Abstract
Purpose of Review
Since its approval by the Unites States Food and Drug Administration (FDA) in 2015, cone-beam breast computed tomography (CBBCT) has gained acceptance among radiologists for breast cancer imaging. This review aims to highlight the advancements and benefits of CBBCT in the diagnostic workup of breast disease. It showcases how CBBCT, including both non-contrast (NC-CBBCT) and contrast-enhanced (CE-CBBCT) protocols, complements and often surpasses the performance of more traditional breast imaging modalities such as mammography and magnetic resonance imaging (MRI).
Recent Findings
Studies in clinical settings have shown CBBCT’s efficacy in detecting and characterizing breast lesions of differing morphologies, including non-mass enhancement and calcifications—tasks that previously required the use of multiple modalities. In addition, CBBCT significantly enhances patient comfort and efficiency, offering quick acquisition times without the discomfort of breast compression. The technology can be utilized for guiding biopsies, planning surgical interventions, and assessing breast density and tumor characteristics, evidence supporting its integration into clinical practice.
Summary
CBBCT holds the potential to shift the imaging paradigm in breast cancer care, indicating a promising future for the modality in terms of enhancing diagnostic accuracy, improving patient experience, and influencing treatment outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most commonly diagnosed cancer and the second leading cause of cancer-related mortality among women in the United States. In 2024, an estimated 313,510 new cases of breast cancer and 42,780 deaths due to the disease are expected [1]. Breast cancer incidence has also increased annually over the past decade, more so in women 50 years in older compared to women younger than 50 years [1]. Despite the increase in breast cancer cases and incidence, breast cancer mortality has decreased by 43%, a decline largely attributed to in early detection and diagnosis by mammography, breast ultrasound, and breast MRI [2]. However, this decline has slowed to 1% over the past decade, stabilizing due to the prevalence of screening mammography and the diagnosis of localized stage and hormone-positive receptor disease. This data suggests that while current imaging technologies have brought us far, there may be a ceiling to the benefits they can provide under existing usage rates and methodology. It underscores the necessity for continuous innovation in cancer detection and treatment planning to overcome this plateau and further reduce mortality rates.
Cone-Beam Breast CT
The dedicated cone-beam breast CT (CBBCT) system was initially approved by the FDA in 2015. The system is equipped with a patient table that has a central opening for the breast to be positioned into the machine. The CBBCT operates by rotating a cone-shaped X-ray beam and a flat-panel detector around the pendant breast, acquiring multiple images in a full 360-degrees scan. The acquired images are then digitally reconstructed into a perfectly isotropic three-dimensional image model of the breast. As of 2024, the CBBCT machine has evolved into its third generation; the most notable changes are a smaller scanner size and footprint and upgrades in the detector, x-ray tube, and x-ray generator that have improved image quality.
We outline the range of technical advantages of CBBCT and explain scenarios where CBBCT is more appropriate for breast imaging workups when compared with existing modalities.
Three-Dimensional Images
Using dedicated viewing software, the 3D reconstructed images of CBBCT can be displayed in orthogonal axial, sagittal, and coronal planes and a 3D maximum intensity projection (MIP). This 3D model effectively eliminates tissue overlap in breast imaging, potentially increasing the detectability of small lesions that may be obscured by glandular tissue in traditional mammography [3, 4]. The benefit of decreased tissue overlap is similar to that touted by non-isotropic digital breast tomosynthesis images, but in the case of CBBCT, the images are truly 3D. The time to interpret each series of CBBCT is similar to a digital breast tomosynthesis stack, and overall time to interpret CBBCT is much less than breast MRI.
High Resolution
The latest CBBCT scanner generates images with a spatial resolution of up to 0.1 mm per pixel in all three dimensions, which nears the resolution of 2D digital mammography (range of 0.05 mm and 0.1 mm per pixel) [5, 6]. Synthetic mammography derived from source projection images of digital breast tomosynthesis has approximately half the resolution of 2D mammography and, in some cases, lower resolution than CBBCT. The 3D high-resolution capability of CBBCT exceeds that of MRI, which attains a maximum resolution of 0.5 to 0.8 mm per pixel at its best performance [7]. Therefore, CBBCT is offered as the potential preeminent modality for breast imaging, offering the highest spatial resolution in 3D.
Absence of Compression
The comfort level of CBBCT system is considered superior to digital mammography, because the imaged breast is not compressed. In a study involving 409 patients, the patients rated their level of discomfort during non-contrast or contrast CBBCT exams and digital mammography exams from 0 to 10 with 0 representing “no pain” and 10 representing “the worst pain.” CBBCT was more comfortable than mammography in patients under than 44 years old and between the ages 45 and 59 (P < 0.05) and in the groups of patients with a BMI above 18.5 (P < 0.05). CBBCT was also more comfortable than mammography in both the fatty-breast and dense-breast groups (P < 0.05) [9].
Rapid Acquisition
The single-scan CBBCT is acquired in 7 s per breast without breast compression, affording new levels of efficiency [8]. The duration of the image acquisition time for CBBCT is comparable to that of a traditional 2D screening mammogram, shorter than that of digital breast tomosynthesis, and significantly shorter than breast MRI, which can be 45 to 60 min for a full-protocol exam and at least 10 min for an abbreviated-protocol exam [10, 11]. In addition to minimizing patient discomfort, this efficiency also notably enhances patient throughput, particularly as a full 3D imaging modality.
Contrast-Enhanced Imaging
Contrast-enhanced CBBCT is an intuitive and straightforward extension of CBBCT imaging. The use of CE-CBBCT has been reported by various researchers in breast cancer diagnosis, pre-operative evaluation, subtype analysis and preliminary AI-based research [12,13,14,15, 16••]. The standard CE-CBBCT protocol includes a pre-contrast scan, followed by injection of a weight-based dose of iodinated contrast media (~ 100 ml) at a rate of approximately 2 ml per second. The post-contrast scan images are acquired between 60 and 120 s after the injection to achieve the optimal enhancement [17, 18].
Radiation Dose
The radiation dose of a non-contrast CBBCT scan ranges from 5.1 to 7.5 mGy, based on recent studies of routine use of CBBCT at researchers’ facilities [16••, 19, 20]. The dose varies depending on breast size and density, but is generally equivalent to the radiation dose from a diagnostic mammography exam, which ranges from 4.3 to 10 mGy [21]. For a CE-CBBCT study, the radiation dose doubles with both pre-contrast and post-contrast scans. Uhlig et al. proposed a clinical strategy that involves acquiring only post-contrast CBBCT images to reduce the radiation dose, while still maintaining diagnostic accuracy comparable to that achieved with both pre- and post-contrast acquisitions [22]. We have used this strategy at multiple centers with success and have not seen a compromise in diagnostic performance. Other technical strategies proposed to reduce the radiation dose including system design modifications and few-view reconstruction [23, 24].
A technical factor comparison is summarized in Table 1. Based on the demonstrated advantages, CBBCT has initiated a potential paradigm shift in the diagnostic workup of breast diseases. We now discuss the various clinical applications and review studies conducted worldwide on the use of CBBCT in diagnostic procedures, image-guided biopsy, treatment planning, and innovative research.
Diagnosis
Studies have found that non-contrast CBBCT generally outperforms digital mammography, and contrast-enhanced CBBCT is exceptionally effective in breast cancer detection in Type C and Type D density breasts, described by the American College of Radiology’s Breasting Imaging & Data Reporting System (BI-RADS). In a study involving 442 histopathologically confirmed breast lesions in 212 patients, NC-CBBCT achieved an overall sensitivity and specificity of 88.2% and 84% across all breast density types, which was higher than digital mammography in the same cohort (84.5% and 81.3%, respectively, P < 0.0001). Specifically, in the BI-RADS density type C and D cohort, NC-CBBCT achieved 89.2% sensitivity and 80.1% specificity, and CE-CBBCT reached 98.7% sensitivity and 85% specificity, compared to digital mammography’s 78.4% sensitivity and 70.1% specificity. Both NC-CBBCT and CE-CBBCT significantly outperformed digital mammography in non-dense and dense breast types (P < 0.0001) [25].
Breast MRI, another 3D breast imaging modality, exhibits over 95% sensitivity for breast cancer detection, but has variable specificity, ranging from 30 to 90%. Studies comparing the sensitivity and specificity of CE-CBBCT and breast MRI have been conducted. Wienbeck et al. suggested that, while CE-CBBCT’s sensitivity was slightly lower than MRI, its specificity was numerically higher. In dense breast tissue, CE-CBBCT can be a valuable diagnostic tool, especially for patients with contraindications for MRI [13]. In Uhlig et al.’s meta-analysis, the pooled sensitivity and specificity of CE-CBBCT were 89.9% and 78.8%, comparable to MRI’s 90% sensitivity and 72% specificity from other meta-analyses [26].
Diagnostic parameter measurements are summarized in Table 2 comparing CBBCT, mammography, and breast MRI.
Non-mass enhancement (NME) is an important imaging characteristic of breast lesions, primarily used in the context of breast MRI, which was previously the only contrast-enhanced breast imaging modality [27]. NME refers to areas in the breast that show enhancement on MRI after the administration of contrast material, but do not meet the criteria for or conform to the shape of a mass. CE-CBBCT allows visualization of abnormal contrast uptake and has the potential to characterize NME similar to breast MRI. Kang et al. conducted an initial study on the characteristics of NME on CBBCT, incorporating enhancement distribution, internal enhancement patterns, Hounsfield unit measurements, and the distribution and morphology of associated calcifications. Their combined diagnostic model utilizing the above indicators achieved the diagnostic sensitivity and specificity of 95.65% and 60.00%, respectively [28].
Background parenchymal enhancement (BPE) significantly impacts the interpretation of breast MRIs, as it can affect lesion visibility and characterization. BPE in CE-CBBCT was compared with MRI in a retrospective study by Ma et al. involving 221 patients. The study found substantial agreement between CE-CBBCT and MRI in the reporting of BPE. The study suggested that the BI-RADS MRI lexicon could be applied to describe BPE levels in CE-CBBCT. However, BPE levels in CE-CBBCT were found to be lower than in MRI (P < 0.001), suggesting that BPE poses less diagnostic interference in CE-CBBCT compared to breast MRI [20].
The capability of CBBCT to detect microcalcifications has been debated since its introduction. Research suggests that CBBCT can detect microcalcifications, though its efficacy varies compared to modalities like digital mammography (DM). Liu et al. compared calcification detection between CBBCT and DM in 115 paired examinations, finding substantial agreement with CBBCT’s sensitivity and specificity of 98.43% and 98.85%, respectively [29]. Neubauer et al. used 21 post-surgery breast specimens to examine the accuracy of microcalcification detection and resection margins among DM, DBT, and CBBCT. CBBCT demonstrated a microcalcification detection accuracy of 94%, comparable to DM’s 98% and significantly better than DBT’s 83%. Although its specificity (60%) was lower than DM’s (99%) and DBT’s (73%), CBBCT showed the best performance in measuring the distance of microcalcifications to the resection margin with the least number of errors [30]. These studies also indicate that CBBCT’s performance in microcalcification detection is expected to improve with technical advancements.
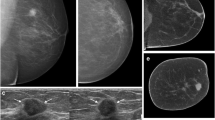
A clinical case is illustrated in the Fig. 1, comparing mammography and breast MRI with NC-CBBCT and CE-CBBCT. CBBCT is capable of characterizing masses, calcifications, and non-mass enhancement (NME) in a single modality, a task that previously required two separate modalities.
A 60 year-old woman with invasive ductal carcinoma (IDC) and ductal carcinoma in situ (DCIS). a Digital mammography shows a cluster of calcifications (hollow arrow) in the lower inner quadrant of the left breast. b Breast MRI shows a single enhancing mass (solid arrow) and an adjacent area of non-mass enhancement (NME) (triangle) in a regional distribution. The mass corresponds to the biopsy-proven IDC, and the NME corresponds to DCIS. c 3D MIP of non-contrast CBBCT shows the mammographically identified calcifications (hollow arrow) and an irregular shaped suspicious mass (solid arrow), not seen on mammography. d 3D MIP of contrast-enhanced CBBCT shows the calcifications (hollow arrow), enhancement of the mass (solid arrow), and the area of non-mass enhancement (triangle) seen on MRI
The Diagnostic Workup
Breast density is a critical factor in assessing the risk of breast cancer and influences decisions about breast cancer detection [31, 32]. As of 2024, 38 states in the United States have legislation requiring that women be notified about their breast density if their mammogram indicates they have dense breasts. Breast density is primarily evaluated visually by mammography, using the ACR BI-RADS breast density categories. As a 3D modality, CBBCT provides both visual and quantitative depictions of breast density. Ma et al. compared CBBCT with digital mammography, with five radiologists visually assessing the breast density of 130 patients according to ACR BI-RADS categories. The study found that CBBCT is in substantial agreement with mammography in density assessment [33]. Liu et al. further investigated the quantitative volumetric density measurement of CBBCT in 216 breasts. Their study found that volumetric density on CBBCT images is positively correlated with visually based BI-RADS density assessments [34]. These initial studies underscore the modality’s potential for breast density reporting in the diagnostic workup.
Imaging plays a critical role in the evaluation of breast cancer. It assists in determining the extent of disease, aids in surgical planning, and helps assess the response to neoadjuvant therapy. Preoperative breast MRI is well-known for its ability to detect additional cancers that may not be visible on mammography and ultrasound. Breast MRI can significantly influence surgical management, recurrence rates, survival, re-excision rates, and early detection of bilateral cancer. MRI provides detailed information about tumor size, multifocality, and chest wall invasion, especially in dense breasts or when other modalities yield unclear results. CE-CBBCT possesses the same feature set as breast MRI, enabling radiologists to fully evaluate the extent of the disease, and is a high-resolution, full 3D modality capable of faster image acquisition.
Siddall et al. compared pre-biopsy CE-CBBCT images with pre-operative MRI to demonstrate that CE-CBBCT has comparable performance in detecting breast cancer multifocality to breast MRI. In selected cases, CE-CBBCT identified lesions, particularly microcalcifications, not seen on MRI [35].
Wienbeck et al. studied 94 lesions from 40 mastectomy specimens to evaluate agreement of lesion size measuring on imaging and the histopathological gold standard. Although the mastectomy specimens were not placed in the designated imaging positions for each modality, the study found that CBBCT yielded smaller absolute size deviations from the gold standard, demonstrating its advantage in determining lesion location in 3D [36]. Later, Wang et al. compared preoperative CE-CBBCT with breast MRI in assessing residual tumor following chemotherapy. Using pathology tumor sizes from 91 patients as the gold standard, the study discovered that CE-CBBCT showed overall smaller discrepancy in tumor size measurement than MRI (median (IQR): 0.24 cm vs. 0.67 cm) when compared to pathology. In cases with residual ductal carcinoma in situ (DCIS) or calcifications and in luminal A and B-type breast cancers, CE-CBBCT demonstrated even less deviation with pathology compared to MRI. In addition, CE-CBBCT demonstrated comparable performance in predicting pathologic complete response, with an AUC of 0.749, compared to an AUC of 0.733 for MRI [16••].
CBBCT-Guided Biopsy
The CBBCT-guided biopsy procedure closely resembles that of MRI-guided biopsy, requiring only minimal additional training for the technologist and the radiologist to familiarize themselves with the biopsy protocol and tools. The CBBCT scanner is equipped with a bracket attachment for image-guided biopsy that is installed and attached through the opening in the table where the breast is positioned during the scan. The bracket stabilizes the breast by compressing it between a third-party grid and backplate set similar to MRI-guided breast biopsy. MRI-compatible vacuum-assisted biopsy probes and localization kits produced by major vendors can be directly used for the CBBCT procedure.
The CBBCT-guided biopsy was first studied by Seifert et al. to compare it with stereotactic biopsy. According to their head-to-head phantom comparison study, both CBBCT and stereotactic biopsy achieved a 100% mass and calcium lesion retrieval rate. The biopsy time ranged from 10 to 20 min for both modalities. The radiation dose for CBBCT biopsy was approximately 50% of that for stereotactic biopsy [37]. Later, Wienbeck et al. reported a comparison study between CBBCT biopsy and stereotactic biopsy on 68 lesions in 65 patients. The average biopsy time was 22.7 ± 8.4 min in the CBBCT biopsy group (31 lesions), significantly faster than the 28.8 ± 9.4 min in the stereotactic biopsy group (37 lesions) [38]. CE-CBBCT-guided biopsy has also been reported by Hoxhaj et al. with both phantom and patient studies in comparison with MRI-guided biopsy. The median total intervention time for CE-CBBCT-guided biopsy was 22 min in phantoms and 29 min in patients, substantially shorter than the 72 min reported for MRI-guided biopsy in the literature [39].
Surgical Planning
CBBCT has recently started to draw attention from breast surgeons due to its rapid imaging, ease of accessibility, ability to detect both calcifications and masses, and accurate 3D measurements. Li et al. reported a retrospective study comparing the tumor-to-gland volume ratio and tumor-to-breast volume ratio between 100 breast-conserving surgery patients and 100 mastectomy patients. The study found that both the tumor-to-gland volume ratio and the tumor-to-breast volume ratio were significantly lower in the breast-conserving surgery group (4.32% and 0.74%, respectively) than in the mastectomy group (10.74% and 1.36%, respectively). They concluded that the tumor-to-gland volume ratio and tumor-to-breast volume ratio measured in CBBCT images, after tumor segmentation and tissue segmentation with 3D measurement tools, can be predictors of the suitability for breast-conserving surgery [40].
Sun et al. reported a pilot study on breast-conserving surgery in patients with extensive calcifications. In their study, patients with enhancing lesions and segmentally distributed calcifications in CE-CBBCT were selected for the procedure. The measurements were first performed on the 3D images to localize the lesion’s volume, followed by the use of radiopaque marking to transfer the imaging measurements onto the patient’s skin. A second CBBCT scan was taken to confirm the surface marking covered the entire lesion before the surgeon proceeded with the lumpectomy. The CBBCT-guided surface localization and oncoplastic breast-conserving surgery were successful in all 11 patients in the study, with negative margins achieved in each case [41•].
Innovative Studies on CBBCT Characteristics
With the rich information provided by NC-CBBCT and CE-CBBCT, innovative studies have utilized the quantitative information extracted from CE-CBBCT enhancement patterns. An early study conducted by Uhlig et al. found that the Hounsfield units in CE-CBBCT images are correlated with breast cancer immunohistochemical subtypes [12]. Zhu et al.’s study discovered that both quantitative and morphological features in CE-CBBCT images can serve as biomarkers to prognosticate HER2 receptor status [42]. Subsequently, Ma et al. identified 11 CE-CBBCT features associated with breast cancer molecular subtypes. They generated two prediction models based on subsets of these features to classify breast cancers between luminal and non-luminal types, and between HER2-enriched and triple-negative types [15].
Recent studies have also highlighted the potential of CE-CBBCT features in radiomics, marking a new frontier in breast imaging and oncology. Zhu et al. analyzed clinical features and 426 radiomic features mined from CE-CBBCT. Radiomic models, along with a clinical model and a combined clinical-radiomic model, were developed to predict axillary lymph node metastasis status. The radiomics model alone achieved an AUC of 0.75 in predicting axillary lymph node status and an AUC of 0.65 in predicting metastatic burden, outperforming both the clinical model (AUC 0.68 and 0.55, respectively) and the combined model (AUC 0.74 and 0.64). A nomogram was developed to predict the risk of axillary lymph node metastasis, combining radiomics features with location and focality as independent predictors [43, 44]. The coverage of lymph nodes varies in the early generations of CBBCT; however, the radiomics approach, coupled with new detector and system designs in the latest generations of CBBCT, has the potential to overcome this limitation [45•].
Conclusions
The advent and evolution of cone-beam breast CT (CBBCT) over recent years signify a groundbreaking advancement in the diagnostic workup of breast diseases. Since its initial FDA approval in 2015, CBBCT, both non-contrast and contrast-enhanced imaging, has demonstrated substantial benefits over traditional imaging modalities, including mammography and MRI. CBBCT possesses the ability to produce high-resolution, three-dimensional images rapidly and without compression and offers unparalleled patient comfort and efficiency, enhancing patient throughput and experience.
Moreover, the clinical applications of CBBCT, ranging from diagnostic procedures and image-guided biopsy to treatment planning and innovative research, highlight its versatility and potential to shift paradigms in breast cancer diagnosis and management. The modality’s capacity for detailed imaging supports a more nuanced understanding of breast diseases, offering insights into breast density, lesion characterization, and even potential surgical outcomes.
As we continue to explore and refine the capabilities of CBBCT, it is imperative to balance technological innovation with patient safety, ensuring that advancements in breast imaging are accessible, effective, and aligned with the ultimate goal of reducing breast cancer mortality. The journey of CBBCT, from its conception to its current state, reflects a promising trajectory towards more precise, patient-centered breast cancer care, marking a significant step forward in the fight against this pervasive disease.
Data Availability
No datasets were generated or analyzed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Siegel RL, et al. Cancer statistics, 2024. CA Cancer J Clin. 2023;73(1):17–48.
Society AC. Breast cancer facts & figures 2022–2024. Atlanta: American Cancer Society; 2022.
O’Connell A, et al. Cone-beam CT for breast imaging: radiation dose, breast coverage, and image quality. AJR Am J Roentgenol. 2010;195(2):496–509.
Boyd NF, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–36.
Huda W, Abrahams RB. X-ray-based medical imaging and resolution. AJR Am J Roentgenol. 2015;204(4):W393–7.
Rangarajan K, et al. Ultra-high resolution, multi-scale, context-aware approach for detection of small cancers on mammography. Sci Rep. 2022;12(1):11622.
Rahbar H, et al. Clinical and technical considerations for high quality breast MRI at 3 Tesla. J Magn Reson Imaging. 2013;37(4):778–90.
Koning Corporation, The Koning Differences. https://www.koninghealth.com/product-solutions/koning-vera-breast-ct. Accessed 27 Jan 2024
Li H, et al. Comparison of comfort between cone beam breast computed tomography and digital mammography. Eur J Radiol. 2019;120: 108674.
Houser M, Barreto D, Mehta A, et al. Current and future directions of breast MRI. J Clin Med. 2021;10(23):5668.
Kwon MR, et al. Breast cancer screening with abbreviated breast MRI: 3-year outcome analysis. Radiology. 2021;299(1):73–83.
Uhlig J, et al. Contrast enhancement on cone-beam breast-CT for discrimination of breast cancer immunohistochemical subtypes. Transl Oncol. 2017;10(6):904–10.
Wienbeck S, et al. Contrast-enhanced cone-beam breast-CT (CBBCT): clinical performance compared to mammography and MRI. Eur Radiol. 2018;28(9):3731–41.
Ma J, et al. Distinguishing benign and malignant lesions on contrast-enhanced breast cone-beam CT with deep learning neural architecture search. Eur J Radiol. 2021;142:109878.
Ma Y, Liu A, O'Connell AM, et al. Contrast-enhanced cone beam breast CT features of breast cancers: Correlation with immunohistochemical receptors and molecular subtypes. Eur Radiol. 2021;31(4):2580–9.
Wang Y, Zhao M, Ma Y, et al. Accuracy of preoperative contrast-enhanced cone beam breast CT in assessment of residual tumor after neoadjuvant chemotherapy: A comparative study with breast MRI. Acad Radiol. 2023;30(9):1805–15. This article describes CBBCT’s superior performance to breast MRI when evaluating tumor size and response to chemotherapy.
Uhlig J, et al. Contrast-enhanced cone-beam breast-CT: analysis of optimal acquisition time for discrimination of breast lesion malignancy. Eur J Radiol. 2018;99:9–16.
Chen JT, Zhou CY, He N, et al. Optimal acquisition time to discriminate between breast cancer subtypes with contrast-enhanced cone-beam CT. Diagn Interv Imaging. 2020;101(6):391–9.
Zhao X, Yang J, Zuo Y, et al. Contrast-enhanced cone-beam breast CT: An analysis of diagnostic value in predicting breast lesion with rim enhancement malignancy. Front Oncol. 2022;12:868975.
Ma Y, et al. Comparison of background parenchymal enhancement (BPE) on contrast-enhanced cone-beam breast CT (CE-CBBCT) and breast MRI. Eur Radiol. 2022;32(8):5773–82.
Miglioretti DL, et al. Radiation-induced breast cancer incidence and mortality from digital mammography screening: a modeling study. Ann Intern Med. 2016;164(4):205–14.
Uhlig J, et al. Pre- and post-contrast versus post-contrast cone-beam breast CT: can we reduce radiation exposure while maintaining diagnostic accuracy? Eur Radiol. 2019;29(6):3141–8.
Xie H, Shan H, Cong W, et al. Deep efficient end-to-end reconstruction (deer) network for few-view breast CT image reconstruction. IEEE Access. 2020;8:196633–46.
Tseng HW, Karellas A, Vedantham S. Dedicated cone-beam breast CT: Data acquisition strategies based on projection angle-dependent normalized glandular dose coefficients. Med Phys. 2023;50(3):1406–17.
He N, et al. The utility of breast cone-beam computed tomography, ultrasound, and digital mammography for detecting malignant breast tumors: a prospective study with 212 patients. Eur J Radiol. 2016;85(2):392–403.
Uhlig J, et al. Diagnostic accuracy of cone-beam breast computed tomography: a systematic review and diagnostic meta-analysis. Eur Radiol. 2019;29(3):1194–202.
D’Orsi CJ, Sickles EA, Mendelson EB, Morris EA, et al. ACR BI-RADS® Atlas, breast imaging reporting and data system. Reston, VA: American College of Radiology; 2013.
Kang W, Zhong W, Su D. The cone-beam breast computed tomography characteristics of breast non-mass enhancement lesions. Acta Radiol. 2021;62(10):1298–308.
Liu A, Ma Y, Yin L, et al. Comparison of malignant calcification identification between breast cone-beam computed tomography and digital mammography. Acta Radiol. 2023;64(3):962–70.
Neubauer C, et al. Accuracy of cone-beam computed tomography, digital mammography and digital breast tomosynthesis for microcalcifications and margins to microcalcifications in breast specimens. Sci Rep. 2022;12(1):17639.
Bodewes FTH, et al. Mammographic breast density and the risk of breast cancer: a systematic review and meta-analysis. Breast. 2022;66:62–8.
Advani SM, et al. Association of breast density with breast cancer risk among women aged 65 years or older by age group and body mass index. JAMA Netw Open. 2021;4(8): e2122810.
Ma Y, et al. A reliability comparison of cone-beam breast computed tomography and mammography: breast density assessment referring to the fifth edition of the BI-RADS atlas. Acad Radiol. 2019;26(6):752–9.
Liu A, et al. Quantitative breast density measurement based on three-dimensional images: a study on cone-beam breast computed tomography. Acta Radiol. 2022;63(8):1023–31.
Siddall, K. Exploring the diagnostic performance of dedicated cone-beam breast CT: Can it be utilized as a substitute for breast MRI? In: SBI ACR breast imaging symposium; 2022. https://www.eventscribe.net/2022/SBIACR2022/fsPopup.asp?efp=VlVWTERNS1kxNTM5OQ&PresentationID=1070475&rnd=0.5330223&mode=presinfo. Accessed 27 Jan 2024.
Wienbeck S, et al. Breast lesion size assessment in mastectomy specimens: correlation of cone-beam breast-CT, digital breast tomosynthesis and full-field digital mammography with histopathology. Medicine. 2019;98(37):e17082.
Seifert PJ. Initial experience with a breast computed tomography guided biopsy system (BCT-GBx) for cone beam breast CT (CBBCT). RSNA; 2013. https://archive.rsna.org/2013/13044191.html. Accessed 27 Jan 2024.
Wienbeck S, Lotz J, Fischer U. Feasibility of vacuum-assisted breast cone-beam CT-guided biopsy and comparison with prone stereotactic biopsy. AJR Am J Roentgenol. 2017;208(5):1154–62.
Hoxhaj A, Sechopoulos I, Mann RM. Contrast-enhanced cone-beam breast CT-guided biopsies in breast phantoms: accuracy, rate of diagnostic success, and total intervention time. EMJ Radiol. 2023;4:38–40.
Li J, Zhong G, Wang K, et al. Tumor-to-gland volume ratio versus tumor-to-breast ratio as measured on CBBCT: Possible predictors of breast-conserving surgery. Cancer Manag Res. 2021;13:4463–71.
Sun Y, He N, Ye F, et al. Cone-beam breast CT-guided surface location facilitates breast-conserving surgery in breast cancer patients with extensive calcifications: A pilot study. Front Surg. 2023;10:1070868. This article describes an exciting potential future application of CBBCT.
Zhu Y, et al. Cone-beam breast CT features associated with HER2/neu overexpression in patients with primary breast cancer. Eur Radiol. 2020;30(5):2731–9.
Zhu, Y., et al., Radiomics in cone-beam breast CT for the prediction of axillary lymph node metastasis in breast cancer: a multi-center multi-device study. Eur Radiol. 2023.
Zhu Y, Ma Y, Zhang Y, et al. Radiomics nomogram for predicting axillary lymph node metastasis-a potential method to address the limitation of axilla coverage in cone-beam breast CT: a bi-center retrospective study. Radiol Med. 2023;128(12):1472–82.
Vedantham S. Contrast-enhanced breast computed tomography: Can lymph node metastasis be predicted from primary tumor? Eur Radiol. 2023. https://doi.org/10.1007/s00330-023-10399-4. Online ahead of print. This article describes an exciting potential future application of CBBCT.
Author information
Authors and Affiliations
Contributions
KS and XZ wrote the main manuscript text and prepared the figure. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
Financial interests: Author B is employed by Koning Corporation. Author A and Author C have received stock options from Koning Corporation.
Human and Animal Rights and Informed Consent.
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Siddall, K., Zhang, X. & O’Connell, A. Emerging Clinical Applications for Cone Beam Breast CT: Changing the Breast Imaging Paradigm. Curr Breast Cancer Rep 16, 134–141 (2024). https://doi.org/10.1007/s12609-024-00535-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12609-024-00535-4