Abstract
Purpose of the Review
Breast lesions of uncertain malignant potential (B3) are a heterogeneous group of diagnostic entities that can be associated with atypia. These are associated with in situ or invasive malignancy in 20–30% of cases. The management of those lesions has been both controversial and challenging for the multidisciplinary teams.
Recent Findings
This is an up-to-date review of the current International Consensus on the management of B3 lesions with emphasis on the practical considerations.
Summary
Through multidisciplinary management pathways, the vast majority of B3 lesions should be managed by vacuum-assisted excision, rather than surgical excision. Exceptions to this pathway and the rationale for surgical excision are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The UK breast screening programme classifies a group of breast lesions of uncertain malignant potential as B3 lesions. This is a heterogeneous category encompassing a number of lesions that may or may not be associated with cytological and/or architectural atypia.
The B3 category includes cellular fibroepithelial lesions (where phyllodes tumour is considered), flat epithelial atypia (FEA), lobular in situ neoplasia (including ALH, LCIS), atypical intraductal epithelial proliferation (AIEDP), intraduct papillomas (with and without atypia), radial scars (with and without atypia) and mucocele-like lesions. A combination of the B3 lesions is known to occur particularly in the spectrum of low nuclear grade neoplasia family (FEA, lobular neoplasia, AIDEP) [1]. Other less common lesions within this category include apocrine atypia, myofibroblastomas, vascular lesions and bland spindle cell lesions.
The proportion of B3 lesions varies across institutions both nationally and internationally. In the UK, the reported rate between 1999 and 2006 was 5% (range 2.417.93%) [2]. In large European series, the B3 rate was reported at 4.5% in Germany [3], 11.9% in Italy [4] and 17% in Switzerland [5]. A recent study of 5750 needle core biopsies in the USA showed an incidence of 8%, of which atypical ductal hyperplasia (ADH) was the commonest lesion (4.3%) [6].
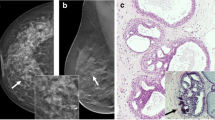
Histologically, some of the B3 entities, such as FEA and AIDEP (Fig. 1a, b), are known to be diagnostically challenging with significant inter and intra observer variation [7]. The reproducibility of diagnosis however is higher among specialist breast pathologists and improves on applying agreed criteria for diagnosis [8].
Histological features of flat epithelial atypia, atypical intraductal epithelial proliferation and marker clip reactions. a Flat epithelial atypia (FEA) with luminal calcifications. There is a dilated terminal duct lobular unit lined by slightly enlarged hyperchromatic cells showing low-grade cytological atypia. The ductal lumina show secretion and round calcification. Note the absence of high-grade cytological atypia. b Atypical intraductal epithelial proliferation (AIDEP). This breast core shows two ducts with architectural atypia showing a cribriform pattern. The intraductal proliferation is incompletely involved by low-grade atypical cells and the lesional size is less than 2 mm and therefore does not amount to ductal carcinoma in situ. c An example of marker clip reaction. The bottom of the figure shows the architecture of a marker clip comprising interconnecting trabecula of acellular eosinophilic material. This is surrounded by a florid foreign body giant cell reaction and chronic inflammation. d A different type of marker clips in VAE core comprising a cystic space lined by histiocytes and foreign body giant cells. The adjacent breast tissue shows fat necrosis consistent with previous sampling
B3 Upgrade Rate
Many studies assessed the upgrade rate to in situ or invasive carcinoma (positive predictive value) following the diagnosis of B3. Overall, the association with malignancy is seen in 20–30% of cases [2, 3, 9•], Table 1. Most of the upgrades are to in situ rather than invasive carcinoma. In other words, the majority of B3 lesions show a final benign diagnosis.
It has long been known that the rate of progression to malignancy is low and occurs over a long period [14]. The cancers that develop in a small proportion of B3 lesions are often of the low-grade hormone receptor–positive type (i.e. good prognosis group). The rationale for further sampling, however, is to exclude coexistent, more advanced disease that could have been missed by the diagnostic core biopsy. The B3 lesions can be heterogeneous co-existing with in situ or invasive carcinoma in only part of the lesion hence the need for further tissue examination. This ensures adequate sampling to exclude associated focal carcinoma. Therefore, vacuum-assisted excision is considered a viable alternative to surgical diagnostic excision as treatment option.
Current Guidelines for Management of B3 Lesions
The management of those lesions has been challenging. Traditionally, B3 lesions are managed by surgical excision. There has been a trend globally to use VAE for the management of B3 lesions with no atypia, such as radial scars and papillomas with no atypia [15]. The B3 lesions with atypia are, however, still managed with diagnostic surgical excision in Europe and the USA.
The first International Consensus Conference on lesions of uncertain malignant potential in the breast recommended VAE as the gold standard for managing the majority of those lesions [16]. The recently published second International Consensus endorsed those recommendations [17••]. Similarly, the UK published imaging guidance [18], followed by detailed pathological guidelines [19••] on the diagnostic criteria, significance and management of each B3 category. The UK guidelines recommend vacuum-assisted excision (VAE) as the gold standard for managing all B3 lesions, except papilloma with atypia, spindle cell lesions and fibroepithelial lesions.
The rationale for opting for a diagnostic excision following the diagnosis of papillary lesion with atypia is to allow pathologists to measure the precise extent of atypia to differentiate between atypia within a papilloma (ADH, < 3 mm) and DCIS within a papilloma (> 3 mm). The diagnosis of phyllodes tumour and its categorisation into benign/borderline/malignant requires the assessment of several histological criteria including the status of the margin (circumscribed vs infiltrative) and adequacy of excision.
It is to be noted that there are some discrepancies between the European and UK recommendations for managing B3 lesions. For example, the International Consensus recommends a surgical excision following an AIDEP diagnosis [17••]. On the other hand, lobular neoplasia on core biopsy without discordant imaging is often managed by imaging follow-up in the USA [20] and this is supported by the National Comprehensive Cancer Network (NCCN) and the American Society of Breast Surgeons (ASBS) guidelines [21••].
Diagnostic Vacuum–Assisted Biopsy vs Vacuum-Assisted Excision
It is important to clarify the use of terminology in relation to the vacuum biopsy device. Vacuum-assisted biopsy (VAB) is where the conventional 14G needle has been replaced with a vacuum biopsy needle (usually 12-9G) and the aim is to obtain a diagnosis. VAB allows sampling a larger amount of tissue (compared with conventional needle core biopsy) to provide enough tissue for the pathologists to make a diagnosis. The purpose is not to excise the whole lesion. Similar to conventional core biopsy, a final B-category is recorded.
Vacuum-assisted excision (VAE) is used to replace surgical diagnostic biopsy. Often, 7 or 8G needle is used and the aim is to obtain plentiful samples to equate to a surgical biopsy with representative sampling. The aim is to take about 4G of tissue which is often estimated by multiplying the number of cores with estimated weight of each core dependent on the needle size [19••]. The advent of vacuum technology has the advantage of adequate tissue sampling without the complications of surgical excision. Small lesions, typically less than 15 mm, may be completely excised by VAE.
Advantages of VAE
There have been concerns regarding the over diagnosis and overtreatment of women within the breast screening programme. Women with a likely outcome of a benign diagnosis should not therefore be exposed to unnecessary surgery.
VAE is a well-tolerated outpatient procedure aiming to sample further tissue to exclude more advanced lesions. Considering that the majority of B3 patients end up with a final benign diagnosis, the technique saves those patients unnecessary surgical excisions. Patients can therefore avoid the unwanted complications of surgery including anaesthetic complications, scarring and difficult follow-up mammographic surveillance due to post-surgical changes.
The prevalent benign operative biopsy rate within the UK has been variable. The minimum standard is 1.5 per 100,000 and target is 1 per 100,000. The outlier units are generally those who have not implemented this pathway. VAE reduces the benign diagnostic biopsy rate and improves the preoperative diagnostic rate without affecting the cancer detection rate.
Marker Clips
The insertion of a marker clip is strongly recommended in the sampling of calcification. If more than one area is being sampled, it is good practice to use a different clip type per area. This will allow accurate identification of the sampled areas particularly if the histological diagnoses are different. Pathological correlation including identifying the marker clip reaction histologically is important to ascertain sampling of the area of interest. The histological appearances of the marker clips vary with the type inserted and these are often associated with a florid inflammatory and foreign body giant cell reaction (Fig. 1c, d).
Recording of Breast Screening Data
Currently, a B coding is provided for all core biopsy diagnoses. VAE is a larger biopsy that is meant to replace diagnostic excision and hence a final B coding is not required. The current National Breast Screening System (NBSS) has been adapted so that this data can now be collected from all the breast screening units providing national data on how B3 lesions are being managed. All the fields related to a B3 diagnosis are now mandatory where the type of B3 lesion is recorded and the presence or absence of atypia noted. The VAE is also recorded on NBSS as a mandatory field. No final B coding is required by pathologists or recorded for those lesions excised by vacuum biopsy [22••]. The NHS Breast Screening Programme are keen to minimise overtreatment in the context of B3 lesions and have created a new KPI (key performance indicator) where all appropriate B3 lesions should be managed with VAE and < 25% of B3 lesions should be managed with surgery.
All 80 screening units within the UK will be audited on a yearly basis and units where the surgical diagnostic biopsy rate is > 25%, the units will have to audit their practice and explain why they have not achieved the target.
Follow-up Following a B3 Diagnosis
One important issue following the diagnosis of B3 lesions, particularly those with atypia, is whether to follow patients up radiologically and the length and frequency of follow-up. There is no hard evidence to guide the duration and frequency of follow-up. The International Consensus paper recommended surveillance following a B3 diagnosis including for non-atypical lesions (papilloma, radial scars) and more frequent surveillance for lobular neoplasia [17••].
The current UK guidelines recommend annual mammographic follow-up for 5 years followed by return to the 3 yearly routine breast cancer screening. This will generate good evidence based data to inform future follow-up strategies.
Radiological Pathological Issues
Terminology
Histologically, the term ‘AIDEP’ is recommended for describing low-grade atypical ductal hyperplasia (ADH) diagnosed on core biopsy and/or diagnostic VAB. The ‘ADH’ terminology is quantitative and can only be used when the area of interest has been thoroughly sampled and the extent of the lesion confirmed to be less than 2 mm [19••]. Lesions larger than 2 mm are designated as ductal carcinoma in situ (DCIS).
Reporting of Atypia
It is recommended that the diagnosis of B3 lesions should include a comment on the presence/absence of atypia. Atypia is associated with a higher risk of upgrade to in situ/invasive carcinoma among all B3 lesions [9•, 13, 23]. The presence of atypia can also alter the management plans. For example; benign papillomas without atypia are managed by VAE whereas those with atypia require diagnostic surgical excisions [19••].
Complete Excision of Lesion
For some B3 lesions, complete excision of the lesion may be done on the first diagnostic VAB and no further abnormality can be identified on VAE examination. This is not uncommon. It is important in this context to confirm that the correct area has been sampled (histological evidence of previous core/VAB biopsy site and/or marker clip reaction).
Adequacy of Excision
Due to the piecemeal nature of the vacuum biopsies, it is not possible for pathologists to comment on the adequacy of excision (e.g. for a radial scar, papilloma). Decisions on adequate excision in those instances will depend on the radiological impression.
Clip Migration
In a small proportion of cases, haematoma leading to clip migration may occur. In those instances, radiological review and documentation is required.
Radiological Pathological Discordance
Radiological pathological correlation is the essence on the B3 management pathway. For discordant lesions (e.g. histological findings do not explain a mass lesion or calcification not identified), a repeat VAB and/or a diagnostic excision may be required. Discussion at the multidisciplinary meeting with careful planning and documentation are therefore important.
Conclusions
The current B3 management pathway is multidisciplinary and recommends the use of VAE as the standard for the majority of B3 lesions. This pathway ensures adequate sampling to exclude coexistent in situ or invasive carcinoma. Yearly mammographic surveillance for 5 years is also recommended for atypical lesions. A separate category for VAE coding on NBSS is now available. Regular auditing and collection of outcome data will allow refining recommendations on the duration and frequency of follow-up.
Abbreviations
- ADH:
-
Atypical ductal hyperplasia
- AIDEP:
-
Atypical intraductal epithelial proliferation
- ALH:
-
Atypical lobular hyperplasia
- ASBS:
-
The American Society of Breast Surgeons
- DCIS:
-
Ductal carcinoma in situ
- FEA:
-
Flat epithelial atypia
- LCIS:
-
Lobular carcinoma in situ
- NBSS:
-
National Breast Screening System
- NCCN:
-
National Comprehensive Cancer Network
- VAB:
-
Vacuum-assisted biopsy
- VAE:
-
Vacuum-assisted excision
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Abdel-Fatah TM, Powe DG, Hodi Z, Reis-Filho JS, Lee AH, Ellis IO. Morphologic and molecular evolutionary pathways of low nuclear grade invasive breast cancers and their putative precursor lesions: further evidence to support the concept of low nuclear grade breast neoplasia family. Am J Surg Pathol. 2008;32(4):513–23.
El-Sayed ME, Rakha EA, Reed J, Lee AH, Evans AJ, Ellis IO. Predictive value of needle core biopsy diagnoses of lesions of uncertain malignant potential (B3) in abnormalities detected by mammographic screening. Histopathology. 2008;53(6):650–7.
Richter-Ehrenstein C, Maak K, Roger S, Ehrenstein T. Lesions of “uncertain malignant potential” in the breast (B3) identified with mammography screening. BMC Cancer. 2018;18(1):829.
Bianchi S, Caini S, Renne G, Cassano E, Ambrogetti D, Cattani MG, et al. Positive predictive value for malignancy on surgical excision of breast lesions of uncertain malignant potential (B3) diagnosed by stereotactic vacuum-assisted needle core biopsy (VANCB): a large multi-institutional study in Italy. Breast. 2011;20(3):264–70.
Saladin C, Haueisen H, Kampmann G, Oehlschlegel C, Seifert B, Rageth L, et al. Lesions with unclear malignant potential (B3) after minimally invasive breast biopsy: evaluation of vacuum biopsies performed in Switzerland and recommended further management. Acta Radiol. 2016;57(7):815–21.
Mooney KL, Bassett LW, Apple SK. Upgrade rates of high-risk breast lesions diagnosed on core needle biopsy: a single-institution experience and literature review. Mod Pathol. 2016;29(12):1471–84.
Rakha EA, Ahmed MA, Aleskandarany MA, Hodi Z, Lee AH, Pinder SE, et al. Diagnostic concordance of breast pathologists: lessons from the National Health Service Breast Screening Programme Pathology External Quality Assurance Scheme. Histopathology. 2017;70(4):632–42.
O’Malley FP, Mohsin SK, Badve S, Bose S, Collins LC, Ennis M, et al. Interobserver reproducibility in the diagnosis of flat epithelial atypia of the breast. Mod Pathol. 2006;19(2):172–9.
• Strachan C, Horgan K, Millican-Slater RA, Shaaban AM, Sharma N. Outcome of a new patient pathway for managing B3 breast lesions by vacuum-assisted biopsy: time to change current UK practice? J Clin Pathol. 2016;69(3):248–54 A large UK screen detected B3 series reporting on a 5-year follow-up of the vacuum-assisted excision pathway in the management of B3 lesions including the upgrade rate on VAE and providing real-life evidence supporting implementing this multidisciplinary pathway.
Nguyen CV, Albarracin CT, Whitman GJ, Lopez A, Sneige N. Atypical ductal hyperplasia in directional vacuum-assisted biopsy of breast microcalcifications: considerations for surgical excision. Ann Surg Oncol. 2011;18(3):752–61.
Rakha EA, Ho BC, Naik V, Sen S, Hamilton LJ, Hodi Z, et al. Outcome of breast lesions diagnosed as lesion of uncertain malignant potential (B3) or suspicious of malignancy (B4) on needle core biopsy, including detailed review of epithelial atypia. Histopathology. 2011;58(4):626–32.
Renshaw AA, Gould EW. Long term clinical follow-up of atypical ductal hyperplasia and lobular carcinoma in situ in breast core needle biopsies. Pathology. 2016;48(1):25–9.
Mayer S, Kayser G, Rucker G, Bogner D, Hirschfeld M, Hug C, et al. Absence of epithelial atypia in B3-lesions of the breast is associated with decreased risk for malignancy. Breast. 2017;31:144–9.
Eusebi V, Feudale e FMP, Micheli A, Conti A, Riva C, et al. Long-term follow-up of in situ carcinoma of the breast. Sem Diagn Pathol. 1994;11(3):223–35.
Bennett IC. The changing role of vacuum-assisted biopsy of the breast: a new prototype of minimally invasive breast surgery. Clinical Breast Cancer. 2017;17(5):323–5.
Rageth CJ, O’Flynn EA, Comstock C, Kurtz C, Kubik R, Madjar H, et al. First iInternational Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions). Breast Cancer Res Treat. 2016;159(2):203–13.
•• Rageth CJ, O’Flynn EAM, Pinker K, Kubik-Huch RA, Mundinger A, Decker T, et al. Second International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions). Breast cancer research and treatment. 2018.The second, recently poublished, consensus supports the recommendations of the first consensus. ADH and phyllodes tumours are managed by surgical excisions. Minimally invasive breast biopsies via VAB are recommended for all other B3 lesions . More frequent imaging surveillance particularly for lobular neoplasia.
Programme. NBS. Clinical guidance for breast cancer screening assessment. NHSBSP publication number 49. 2016;Fourth edition.
•• Pinder SE, Shaaban A, Deb R, Desai A, Gandhi A, Lee AHS, et al. NHS Breast Screening multidisciplinary working group guidelines for the diagnosis and management of breast lesions of uncertain malignant potential on core biopsy (B3 lesions). Clin Radiol. 2018;73(8):682–692. These are the current comprehensive B3 pathology guidelines produced by the NHSBSP B3 Writing Group. The paper provided an overview of the diagnostic criteria and management recommendations of B3 catregoris, lesion by lesion, including diagramatic representation of the management pathways within a multidicplinary framework. VAE is recommended for further sampling of all B3 lesions diagnosed on conventional core or VAB in all categories except: papilloma with atypia, cellular fibroepithelial lesions, B3 spindle cell lesions, vascular lesions and other rare lesions such as myofibroblastomas and apocrine adenosis. The document provides guidance on the adequate weight of VAE samples (4 g) and the approximate number of cores required depending on needle gauge and make of the vacuum-assisted device.
Calhoun BC. Core needle biopsy of the breast: an evaluation of contemporary data. Surg Pathol Clin 2018;11(1):1–16, 1.
•• Schnitt SJ. Problematic issues in breast core needle biopsies. Mod Pathol 2019. A recently published useful review of the types of B3 lesions, challenges in their core biopsy diagnosis and update on the current management strategies in the US. ADH is managed by surgical excision. ALH/LCIS is managed by either radiological follow-up/VAE or surgical excision. The latter is performed if there is radiological-pathological discordance or if other high-risk lesions are present.
•• NHS Breast Screening Programme (BSP) Clinical guidelines for breast cancer screening assessment Guidance: Breast screening: how to record vacuum-assisted excisions 2018. Available from: https://www.gov.uk/government/publications/breast-screening-how-to-record-vacuum-assisted-excisions/breast-screening-how-to-record-vacuum-assisted-excisions. Recently published UK Breast Screening guidelines on recording vacuum biopsies on NBSS. VAB biopsies require a final B coding, whereas no B coding is given for VAE since it is equivelant to a diagnostic excision. The type of B3 lesions is now mandatory and the presence/absence of atypia is also recorded.
Rakha EA, Shaaban AM, Haider SA, Jenkins J, Menon S, Johnson C, et al. Outcome of pure mucocele-like lesions diagnosed on breast core biopsy. Histopathology. 2013;62(6):894–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Breast Cancer Imaging and Screening
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Shaaban, A.M., Sharma, N. Management of B3 Lesions—Practical Issues. Curr Breast Cancer Rep 11, 83–88 (2019). https://doi.org/10.1007/s12609-019-0310-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12609-019-0310-6