Abstract
Obesity is one of the most important known preventable causes of cancer, accounting for up to 20% of cancer deaths in women. Obese women have increased risk of dying from breast cancer as well as an increased risk of distant metastasis. Metabolic Syndrome (MetSyn) is a group of metabolic conditions that include 1) abdominal obesity, 2) atherogenic dyslipidemia, 3) elevated blood pressure, and 4) insulin resistance. MetSyn is known to promote the development of cardiovascular disease and diabetes and may be associated with increased breast cancer risk. Emerging evidence supports an association between mammary adipocytes and their secreted adipocytokines and breast cancer initiation and progression. Metformin (1,1-dimethylbiguanide hydrochloride) is a drug used to treat type 2 diabetes and MetSyn. We review the potential association between MetSyn in promoting breast cancer and emerging evidence for the use of metformin in cancer prevention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a known preventable cause of cancer, accounting for up to 20% of cancer deaths in women, with the highest body mass index (BMI) category (BMI > 40 kg/m2) conferring higher risk [1]. Previous studies have shown an association between postmenopausal breast cancer risk and excessive body weight, and this association is increased in women with a positive family history of breast cancer [2, 3]. In addition, women who are obese have an increased risk of dying from breast cancer as well as an increased risk of distant metastasis [4, 5]. Studies investigating the molecular basis for the association between breast cancer and increased adiposity have focused on differences in circulating hormones, such as estrogen and insulin, in normal-weight versus obese individuals. However, emerging studies provide evidence that mammary adipocytes and their secreted adipocytokines may influence breast cancer proliferation and invasion [6, 7]. Metformin belongs to the biguanide class of oral hypoglycemic agents that is prescribed to over 120 million type 2 diabetes patients worldwide. Recent epidemiologic studies and studies in women with breast cancer provide evidence that metformin may reduce cancer risk and/or improve cancer prognosis. Work by Zakikhani et al. [8] demonstrated that metformin inhibits the growth of breast cancer cells in an AMPK-dependent manner. Several tumor suppressors are involved in the AMPK signaling network, and activated AMPK results in suppression of cell proliferation in normal and tumor cells in both in vitro and in vivo studies. Metformin regulates the AMPK/mTOR pathway, which is implicated in the control of protein synthesis and cell proliferation. Recent evidence suggests that metformin may also regulate stem cell turnover via modulation of cytokine signaling. Taken together, these studies provide support for future clinical studies using metformin for either breast cancer prevention or in combination with cytotoxic chemotherapy.
Metabolic Syndrome: Definition, Diagnosis, and Treatment
What is the Metabolic Syndrome?
The Metabolic Syndrome (MetSyn) is characterized by a group of metabolic risk factors occurring in an individual. These metabolic risk factors include abdominal obesity, atherogenic dyslipidemia (high triglycerides, low high-density lipoprotein [HDL] cholesterol and high low-density lipoprotein [LDL] cholesterol), elevated blood pressure, insulin resistance or glucose intolerance, a proinflammatory state (eg, elevated C-reactive protein), and a prothrombotic state (eg, high fibrinogen or plasminogen activator inhibitor-1) [9, 10]. Individuals with the MetSyn are at increased risk of coronary heart disease, stroke, peripheral vascular disease, and type 2 diabetes. It is estimated that over 50 million individuals living in the United States have MetSyn. The dominant underlying risk factors for this syndrome appear to be abdominal obesity and insulin resistance [9, 10]. Additional risk factors for MetSyn include physical inactivity, aging, polycystic ovarian disease, and a genetic predisposition to insulin resistance. Acquired risk factors for MetSyn include central adiposity and physical inactivity, both of which can elicit insulin resistance and the MetSyn [9, 10]. Most people with insulin resistance have abdominal obesity [9, 10]. The biologic relationships at the molecular level between insulin resistance and metabolic risk factors are not fully understood and appear to be complex.
Diagnosis of the MetSyn
There are no well-accepted criteria for diagnosing MetSyn [9, 10]. The criteria proposed by the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III), with minor modifications, are currently recommended for diagnosis of MetSyn [9, 10]. The American Heart Association (AHA) and the National Heart, Lung, and Blood Institute (NHLBI) recommend that MetSyn be identified as the presence of three or more of the following components: 1) elevated waist circumference (men ≥ 40 inches [102 cm], women ≥ 35 inches [88 cm]; 2) elevated triglycerides (≥ 150 mg/dL); 3) reduced HDL cholesterol (men < 40 mg/dL, women < 50 mg/dL); 4) elevated blood pressure (≥ 130/85 mm Hg); 5) elevated fasting glucose (≥100 mg/dL).
Biologic Overlap of the MetSyn, type 2 Diabetes, and Cardiovascular Disease
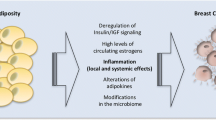
There is significant overlap between MetSyn, Type 2 diabetes, and cardiovascular disease (CVD) [12]. Obesity has direct and indirect effects on CVD, as proinflammatory and prothrombotic factors are produced by visceral fat, and a number of adipocytokines contribute to cardiovascular and diabetes risk [12]. Excessive adipose tissue contributes to insulin resistance through increased release of free fatty acids and cytokines such as tumor necrosis factor-α (TNF-α), and decreased production of adiponectin, an insulin sensitizer [13–17]. Excess adiposity also results in a proinflammatory state owing to increased production of inflammatory cytokines (ie, leptin) and promotes inter-organ interactions involving cytokines and inflammatory mediators and contributes to insulin resistance [18].
Increased insulin resistance results in elevated glucose levels, which can be directly cytotoxic and proinflammatory [11, 19–21]. Elevated glucose levels can contribute to atherosclerosis, such as via advanced glycation products [19, 20, 22]. Probable causes of endothelial dysfunction include elevated glucose, altered lipid profiles, obesity-associated proinflammatory cytokines such as interleukin-6 (IL6), and, possibly, oxidative stress [11, 12]. Effects of exercise include increased lipoprotein lipase, reduced plasma triglycerides, elevated HDL, improved glucose tolerance, heart function and oxygen uptake, and lower blood pressure [23].
Genetic, Epigenetic, and Environmental Factors
All MetSyn traits, such as obesity, dyslipidemia, and type 2 diabetes, are strongly influenced by genetic factors [24], but estimates of inherited risk factors are approximate and frequently make assumptions, such as the absence of gene–environment and gene–gene interactions. In addition, inherited risk estimates reflect both the population and environment [25]. Linkage analysis studies that began in the early 1990s to identify genes contributing to the MetSyn traits have been only modestly successful [11]. The genetic loci identified to date from genome-wide association (GWA) studies explain less than 10% of the population variance of MetSyn traits. Given the high number of individual who carry MetSyn traits, it seems that the GWA study results have only mapped a tiny fraction of their genetic components. This raises the possibility that MetSyn is underpinned by hundreds of genes, each with modest effects, and by many rare mutations. Genes with such modest effects will be difficult to study using standard genetic approaches [11].
Epigenetics and maternal nutrition appear to be important environmental risk factors for MetSyn. Many epidemiologic studies provide evidence that early life stressors, such as poor maternal nutrition, maternal obesity, and rapid postnatal weight gain, can program metabolic adaptations that lead to adult-onset MetSyn [26]. Mechanistic studies in rats and mice have confirmed these associations and have revealed evidence of both metabolic and structural programming [26, 27]. Interestingly, some metabolic traits resulting from poor maternal nutrition can be transmitted to subsequent generations, suggesting the possibility of epigenetic changes maintained during meiosis, as is observed for the agouti coat-color variants in mice [28]. Studies are ongoing by a number of investigators investigating the relationship between early life stressors, environmental toxicants, and MetSyn. One such study is the Newborn Epigenetics Study (NEST) being conducted by Hoyo et al. [29]. It is testing for the effect of maternal nutrition, smoking, and exposure to environmental toxicants in epigenetic imprinting in the offspring [29].
Environmental influences also play a major part in MetSyn [11]. Clearly, a high-calorie diet and a sedentary lifestyle are primary environmental contributors. Environmental factors are difficult to study in humans and epigenetic studies provide evidence that effects of in utero exposures may be transmitted for multiple generations. Furthermore, even if prenatal exposures, diet, and exercise could be accurately assessed, environment–genetic and epigenetic–genetic interactions would be difficult to study [11]. Consequently, few human studies have as yet attempted to tackle gene–gene and gene–environment interactions, and have focused instead on single candidate genes [11]. Whether classical genetic and molecular biology approaches can address these complex interactions is unclear [11].
AHA Recommendation for Managing The MetSyn
The AHA has developed recommendations for managing the MetSyn [9, 10]. The primary goal is to reduce the risk for CVD and type 2 diabetes. First-line therapy is to reduce the major risk factors for cardiovascular disease: stopping smoking and reducing LDL cholesterol, blood pressure, and glucose levels to the recommended levels [9, 10]. Lifestyle therapies are the first-line interventions to reduce the MetSyn risk factors. These lifestyle interventions include 1) weight loss to achieve a BMI < 25 kg/m2; 2) increased physical activity, with a goal of at least 30 min of moderate-intensity activity on most days of the week; and 3) healthy eating, including portion control and reduced intake of saturated fat and trans fat [9, 10].
Role of Metformin in Individuals with The MetSyn
In the Diabetes Prevention Program trial, metformin reduced the risk of incident diabetes [30] and the MetSyn [31] in individuals with impaired fasting glucose and impaired glucose tolerance. In this study, 3234 nondiabetic persons with elevated fasting and post-load plasma glucose concentrations were assigned to metformin (850 mg twice daily) or a lifestyle-modification program. The average follow-up was 2.8 years. The lifestyle intervention reduced the incidence by 58% (95% CI, 48%–66%) and metformin by 31% (95% C, 17%–43%), as compared with placebo; the lifestyle intervention was significantly more effective than metformin. In a 10-year follow-up this study, it was found that prevention or delay of diabetes with lifestyle intervention or metformin could persist for at least 10 years [32]. In patients with the MetSyn but normal glucose tolerance, metformin has been shown to improve endothelial function [33, 34]. In women with polycystic ovarian syndrome (PCOS), multiple short-term studies of 1 year or less have reported significant improvements in the metabolic profile with administration of metformin [35, 36].
Adverse Effects of Metformin
In general, metformin is well tolerated. The most common adverse effect of metformin is gastrointestinal upset, including diarrhea, nausea, and vomiting. In a clinical trial of 286 subjects, 53.2% of the 141 given immediate-release metformin (as opposed to placebo) reported diarrhea, versus 11.7% for placebo, and 25.5% reported nausea/vomiting, versus 8.3% for those on placebo [37, 38]. The most serious potential adverse effect of metformin use is lactic acidosis [37–39]. Lactate uptake by the liver is diminished with metformin administration because lactate is a substrate for hepatic gluconeogenesis, a process that metformin inhibits. In healthy individuals, lactate is excreted by the kidneys and no significant elevation in plasma lactate is observed [37]. However, in individuals with impaired renal function, clearance of metformin and lactate is reduced, leading to increased levels of both, and possibly causing lactic acidosis due to a buildup of lactic acid [37]. Other contraindications include alcoholism (due to depletion of NAD + stores), heart failure, and respiratory disease (due to inadequate oxygenation of tissues). Metformin has also been reported to decrease the blood levels of thyroid-stimulating hormone in patients with hypothyroidism [40]. Long-term use of metformin is associated with increased homocysteine levels [41] and malabsorption of vitamin B12 [42, 43]. Higher doses and prolonged use of metformin are associated with an increased incidence of vitamin B12 deficiency [44].
Biologic Activity of Adipose Tissue
Adipose Tissue as An Endocrine Organ
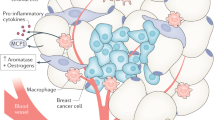
Adipose tissue is an active endocrine organ, secreting fatty acids and peptide hormones or cytokines, collectively called adipocytokines. These biologically active factors act locally on the breast or peripherally to influence multiple processes, including glucose and fatty acid metabolism, insulin signaling, adipocyte differentiation, and inflammation. Accumulating evidence suggests that obesity, a condition characterized by an increase in adipocyte size and number, alters adipocytokine secretion and increases angiogenesis and is a risk factor for breast cancer initiation and recurrence.
Obese individuals typically have elevated levels of circulating leptin, a hormone associated with appetite suppression and energy expenditure. Identifying a causal link between elevated leptin levels and breast cancer initiation and progression has been complicated by conflicting results, but multiple studies provide evidence that leptin has proliferative and proangiogenic effects. Leptin increases proliferation of human breast cancer cell lines [45, 46]. Obese mice deficient in either leptin or the leptin receptor have a reduced occurrence of mammary tumors compared to lean control mice [47, 48], and obese mice lacking leptin or the leptin receptor display defects in mammary gland morphogenesis, implicating leptin in normal mammary tissue development [49]. Leptin signaling promotes expression of vascular endothelial growth factor (VEGF) and VEGFR2, indicating that leptin may stimulate tumor-related angiogenesis [49]. In addition to acting through its own receptor, leptin has been shown to induce ligand-independent activation of estrogen receptor and promote aromatase activity [50, 51]. Taken together, these studies suggest that leptin signaling promotes cell proliferation and angiogenesis.
Adipose tissue is also a major source of hepatocyte growth factor (HGF), another adipocytokine that, like leptin, is elevated in obesity and promotes both cell proliferation and angiogenesis [52]. HGF increases the migration and invasiveness of MDA-MB-231 human breast cancer cells in vitro and also stimulates VEGF-dependent or independent tumor angiogenesis [53, 54]. Several other adipocytokines promote angiogenesis as well, including heparin-binding epidermal growth factor-like growth factor (HB-EGF), TNF-α, and IL-6 (Table 1), providing evidence that increases in the levels of these adipocytokines may also be associated with obesity-related cancers.
Adipocytokines and Breast Cancer Initiation
Excessive adiposity predicts poor survival in women with both estrogen receptor-positive and estrogen receptor-negative breast cancer. Women who exercise and lose 10–15 lb following the diagnosis of breast cancer have a twofold reduction in breast cancer recurrence [2]. Furthermore, women with a BMI >25 kg/m2 have a statistically increased risk of developing precancerous breast changes relative to women who are not overweight (BMI < 25 kg/m2) [1]. As a result, it has been long suspected that mammary adipose stromal cells may play a role in promoting the aggressive behavior of breast cancers, but the molecular contribution of adipose tissue to ER-negative mammary carcinogenesis remains unknown. Until recently, obesity-related breast cancer initiation and recurrence has thought to have been due to estrogen production by adipose tissue. However, there is recent evidence that suggests that changes in adipose cell- or stroma-secreted adipocytokines may also be critical drivers of breast cancer initiation and progression. As detailed above, adipocytokines can affect cell proliferation, apoptosis, invasion, and angiogenesis. Although numerous adipocytokines have been identified, the effects of only a few in promoting or inhibiting mammary tumor growth have been extensively studied to date. These include leptin, HGF, and adiponectin.
Molecular Mechanism of Metformin Action
Metformin, Gluconeogenesis, and Insulin Signaling
Metformin belongs to the biguanide class of oral hypoglycemic agents that are prescribed to over 120 million type 2 diabetic patients worldwide. It is generally agreed that metformin reduces fasting plasma glucose concentrations by reducing rates of hepatic glucose production; its effect on the relative contributions of hepatic glycogenolysis and gluconeogenesis remains controversial. Metformin reduces levels of circulating glucose, increases insulin sensitivity, and reduces insulin resistance–associated hyperinsulinemia. Metformin is known to reduce the level of plasma insulin; however, this is thought to be secondary to a reduction in circulating glucose. At the level of cell signaling, several mechanisms of metformin action have been proposed; the most important one relates to the activation of AMPK.
Metformin and Regulation of Cancer Signaling Pathways
Population studies suggest that metformin decreases the incidence of cancer and cancer-related mortality in diabetic patients [55–58]. Hyperinsulinemia and insulin resistance are associated with poor breast cancer outcomes and recent evidence suggests that insulin can promote tumorigenesis via a direct effect on epithelial tissues, or indirectly by affecting the levels of insulin-like growth factors (IGFs) and adipocytokines. In a retrospective study of women who received neoadjuvant chemotherapy for breast cancer, diabetic cancer patients receiving metformin during their neoadjuvant chemotherapy had a higher pathologic complete response rate than diabetic patients not receiving metformin (24% vs 8%; P = 0.007) [59].
The antineoplastic effects of Metformin in breast cancer are supported by studies demonstrating that metformin inhibits the growth of breast cancer cells [8, 60–68]. Work by Zakikhani et al. [8] demonstrated that metformin inhibits the growth of breast cancer cells in an AMPK-dependent manner. Several tumor suppressors are involved in the AMPK signaling network, and activated AMPK results in suppression of cell proliferation in normal and tumor cells in both in vitro and in vivo studies (Fig. 1). Metformin regulates the AMPK/mTOR pathway, which is implicated in the control of protein synthesis and cell proliferation. High glucose inhibits AMPK. Metformin activates AMPK, and activated AMPK, inhibits mTOR and pACC. IRS-1 phosphorylates Akt [8, 69–71]. Akt/mTOR mobilizes glucose transporters to the cell surface to enhance glucose uptake [8, 69–71]. IRS-1 is subject to negative feedback regulation in response to Akt/mTOR activation (phosphorylation of IRS-1 at Ser612 by mTOR inhibits IRS-1 tyrosine activation) [8, 69–71]. However, loss of this negative feedback promotes continued activation of IRS1-pSer612, p-Akt, and p-mTor and predicts poor prognosis [72].
Epidemiologic and Clinical Trials
Recent pilot epidemiologic studies carried out using population registries provide evidence that metformin may reduce cancer risk and/or improve cancer prognosis. Metformin was associated with a decreased risk of cancer in patients with type 2 diabetes in two initial observational studies reported by Evans et al. [73] and Libby et al. [74]; however, the authors did not provide detailed information on the risk of breast cancer. In a third epidemiologic study, reported by Bowker et al. [75], users of metformin had significantly decreased cancer-related mortality compared with users of either sulfonylureas or insulin. In contrast, Currie et al. [76], in a subgroup analysis of a retrospective cohort study, observed no decrease in breast cancer risk in women taking metformin. Landman et al. [77] reported a lower cancer-related mortality for metformin users compared with that for nonusers. Using the UK-based General Practice Research Database, Bodmer et al. [78] conducted a nested case–control analysis among 22,621 female users of oral antidiabetes drugs with Type 2 diabetes. The multivariate conditional logistic regression analyses were adjusted for use of oral antidiabetes drugs, insulin, estrogens, smoking, BMI, diabetes duration, and HbA1c [78]. In this study, decreased risk of breast cancer was observed in female patients with type 2 diabetes using metformin on a long-term basis [78]. Female diabetic patients receiving neoadjuvant chemotherapy for breast cancer were reported to have a higher complete pathologic response rate if they also used metformin compared with those not using metformin [79]. In this study, Jiralerspong et al. [79] observed that diabetic patients with breast cancer treated with metformin experienced higher pathologic complete response (pCR) rates with neoadjuvant chemotherapy than controls. This was a retrospective analysis of 2592 women, including 157 women with diabetes, treated with neoadjuvant chemotherapy for early stage or locally advanced breast cancer between 1990 and 2007. Diabetic patients treated with metformin experienced a pCR rate of 24%, which was significantly greater than the pCR rate in diabetic women not treated with metformin (8%; P < 0.001) and numerically (but not statistically) greater than the pCR rate in women without diabetes (16%; P = 0.10). In multivariate models adjusting for BMI, stage, tumor grade, hormone receptor and human epidermal growth factor receptor 2 status, age, presence of diabetes, and use of neoadjuvant taxanes, metformin use remained an independent predictor of pCR with an odds ratio of 2.95 (95% CI, 1.07–8.07; P = 0.04). In this model, metformin use was a better predictor of pCR than were established features such as tumor grade, hormone receptor status, and human epidermal growth factor receptor 2/neu over expression.
Emerging role of Metformin and Cancer Stem Cells
Breast cancers are a heterogeneous disease and many breast cancers are composed of multiple cell types with different phenotypic and molecular properties. Cancer stem-like cells (CSCs) are a highly tumorigenic cell type frequently found in clinically aggressive, chemotherapy-resistant breast cancer. The origins of CSCs and their relationships to non-stem cancer cells (NSCCs) are poorly understood. In in vitro studies, CSCs are generated from non-transformed cells at a specific time during the transformation process, but CSC formation is not required for transformation. The adipocytokine IL-6 is sufficient to convert NSCCs to CSCs in breast cell lines and human breast tumors. Thus, it is postulated that tumor heterogeneity involves a dynamic equilibrium between CSCs and NSCCs mediated by IL-6 and activation of the inflammatory feedback. This dynamic equilibrium provides an additional rationale for combining conventional chemotherapy with metformin, which selectively inhibits CSCs. Recent studies provide evidence that metformin may affect cancer stem cells. Iliopoulous et al. [80] recently found that the inducible formation of CSCs and their dynamic equilibrium with NSCCs is modulated by metformin. Metformin was also shown to affect tumor growth factor-β (TGF-β)–induced epithelial to mesenchymal transition (EMT) [81] and suppressed the TGF-β oncogenic micro-RNA (mi-RNA) mi-181A [82]. Together, these observations provide evidence that metformin may have activity against CSCs and provide prevention against aggressive breast cancer and have synergy with cytotoxic chemotherapy.
Conclusions
Taken together, current evidence suggests that metformin may have beneficial effects on breast cancer prevention and outcome. Metformin is an inexpensive and safe drug with minimal toxicity. Minor gastrointestinal upset is the most common toxicity, leading to cessation of therapy in approximately 10% of individuals. Lactic acidosis is a rare complication. Currently, many investigators are developing a large-scale trial of metformin in the adjuvant breast cancer setting and also as a prevention agent for breast cancer. In these studies, it will be critical explore whether any beneficial effect is seen across a broad group of patients with breast cancer (consistent with a direct effect of metformin on AMPK) or whether beneficial effects are restricted to women with hyperinsulinemia, to those whose tumors are insulin receptor positive, or to those whose insulin levels fall in response to metformin treatment (consistent with an indirect effect of metformin acting through an insulin-mediated mechanism). Recent studies providing evidence for metformin in modulating IL-6 signaling and CSCs provide additional evidence that metformin may be synergistic with cytotoxic chemotherapy and provide potential prevention against aggressive breast cancers.
References
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38.
Carpenter CL, Ross RK, Paganini-Hill A, Bernstein L. Effect of family history, obesity and exercise on breast cancer risk among postmenopausal women. Int J Cancer. 2003;106:96–102.
Seewaldt VL, Goldenberg V, Jones LW, et al. Overweight and obese perimenopausal and postmenopausal women exhibit increased abnormal mammary epithelial cytology. Cancer Epidemiol Biomarkers Prev. 2007;16:613–6.
Ewertz M, Jensen M-B, Gunnarsdottir K, Cold S (2009) Effect of Obesity on Prognosis after Early Breast Cancer. Cancer Res 69(24 Suppl):Abstract 18
Berclaz G, Li S, Price KN, et al. Body mass index as a prognostic feature in operable breast cancer: the International Breast Cancer Study Group experience. Ann Oncol. 2004;15:875–84.
Lorincz AM, Sukumar S. Molecular links between obesity and breast cancer. Endocr Relat Cancer. 2006;13:279–92.
Iyengar P, Combs TP, Shah SJ, et al. Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of anti-apoptotic transcriptional programs and proto-oncogene stabilization. Oncogene. 2003;22:6408–23.
Zakikhani M, Dowling R, Fantus IG, et al. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–73.
Grundy SM, Brewer Jr HB, Cleeman JI, et al. Definition of Metabolic Syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation. 2004;109:433–8.
American Heart Association: Metabolic Syndrome. Available at http://www.heart.org/HEARTORG/Conditions/More/MetabolicSyndrome/Metabolic-Syndrome_UCM_002080_SubHomePage.jsp. Accessed April 2011.
Lusis AJ, Attie AD, Reue K. Metabolic syndrome: From epidemiology to systems biology. Nat Rev Genetics. 2008;9:819–30.
Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–80.
Doria A, Patti ME, Kahn CR. The emerging genetic architecture of type 2 diabetes. Cell Metab. 2008;8:186–200.
Wajchenberg BL, Giannella-Neto D, da Silva ME, Santos RF. Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm Metab Res. 2002;34:616–21.
Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–56.
Chibalin AV, Leng Y, Vieira E, et al. Downregulation of diacylglycerol kinase delta contributes to hyperglycemia-induced insulin resistance. Cell. 2008;132:375–86.
Stratford S, Hoehn KL, Liu F, Summers SA. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J Biol Chem. 2004;279:36608–15.
Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nature Rev Mol Cell Biol. 2008;9:193–205.
Hsueh W, Abel ED, Breslow JL, et al. Recipes for creating animal models of diabetic cardiovascular disease. Circ Res. 2007;100:1415–27.
Hudson BI, Hofmann MA, Bucciarelli L, et al. Glycation and diabetes: The RAGE connection. Current Science. 2002;83:1515–21.
Sandhu MS, Waterworth DM, Debenham SL, et al. LDL-cholesterol concentrations: a genome-wide association study. Lancet. 2008;371:483–91.
Saxena R, Voight BF, Lyssenko V, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–6.
Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–28.
(2002) The Genetic Basis of Common Diseases. In: King RA, Rotter JI, Motulsky AG (eds.), New York: Oxford University Press
Visscher PM, Hill WG, Wray NR. Heritability in the genomics era—concepts and misconceptions. Nature Rev Genet. 2008;9:255–66.
Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–6.
Bruce KD, Cagampang FR. Epigenetic priming of the metabolic syndrome. Toxicol Mechanisms Methods. 2011;21:353–61.
Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nature Rev Genet. 2007;8:253–62.
Hoyo C, Murtha AP, Schildkraut JM et al. (2011) Folic acid supplementation before and during pregnancy in the Newborn Epigentetics STudy (NEST). BMC Pub Health 11: article 46
Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
Orchard TJ, Temprosa M, Goldberg R, et al. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med. 2005;142:611–9.
Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–86.
de Aguiar LG, Bahia LR, Villela N, et al. Metformin improves endothelial vascular reactivity in first-degree relatives of type 2 diabetic patients with metabolic syndrome and normal glucose tolerance. Diabetes Care. 2006;29:1083–9.
Vitale C, Mercuro G, Cornoldi A, et al. Metformin improves endothelial function in patients with metabolic syndrome. J Intern Med. 2005;258:250–6.
Goldenberg N, Glueck CJ, Loftspring M, et al. Metformin-diet benefits in women with polycystic ovary syndrome in the bottom and top quintiles for insulin resistance. Metabolism. 2005;54:113–21.
Lord J, Thomas R, Fox B, et al. The effect of metformin on fat distribution and the metabolic syndrome in women with polycystic ovary syndrome—a randomised, double-blind, placebo-controlled trial. BJOG. 2006;113:817–24.
Shegem NS, Nasir AM, Jbour AK, et al. Effects of short term metformin administration on androgens in normal men. Saudi Med J. 2002;23:934–7.
Drug Facts and Comparisons. In: Wickersham RM, Novak KK (eds.), St. Louis, MO: Wolters Kluwer Health, Inc.; 2005
Salpeter S, Greyber E, Pasternak G, Salpeter E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus: systematic review and meta-analysis. Arch Intern Med. 2003;163:2594–25602.
Cappelli C, Rotondi M, Pirola I, et al. TSH-lowering effect of metformin in type 2 diabetic patients: differences between euthyroid, untreated hypothyroid, and euthyroid on L-T4 therapy patients. Diabetes Care. 2009;32:1589–90.
Wulffele MG, Kooy A, Lehert P, et al. Effects of short-term treatment with metformin on serum concentrations of homocysteine, folate and vitamin B12 in type 2 diabetes mellitus: a randomized, placebo-controlled trial. J Intern Med. 2003;254:455–63.
Andrès E, Noel E, Goichot B. Metformin-associated vitamin B12 deficiency. Arch Intern Med. 2002;162:2251–2.
Gilligan M. Metformin and vitamin B12 deficiency. Arch Intern Med. 2002;162:484–5.
de Jager J, Kooy A, Lehert P et al. (2010) Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial. BMJ 340:article c2181
Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer. 2007;14:189–206.
Dieudonne M-N, Machinal-Quelin F, Serazin-Leroy V, et al. Leptin mediates a proliferative response in human MCF7 breast cancer cells. Biochem Biophys Res Commun. 2002;293:622–8.
Frankenberry KA, Skinner H, Somasunder P, et al. Leptin receptor expression and cell signaling in breast cancer. Int J Oncol. 2006;28:985–93.
Cleary MP, Phillips FC, Getzin SC, et al. Genetically obeses MMTV-TGF-alpha/Lep(ob) Lep(ob) female mice do not develop mammary tumors. Breast Cancer Res Treat. 2003;77:205–15.
Cleary MP, Juneja SC, Phillips FC, et al. Leptin receptor-deficient MMTV-TGF-alpha/Let(db) Lepr(db) female mice do not develop ocnogene-induced tumors. Exp Biol Med. 2004;229:182–93.
Hu X, Juneja SC, Maihle NJ, Cleary MGP. Leptin—a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst. 2002;94:1704–11.
Gonzalez RR, Cherfils S, Escobar M, et al. Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type 2 (VEGF-R2). J Biol Chem. 2006;281:26320–8.
Catalano S, Mauro L, Giordano C, et al. Leptin induces, via ERK1/ERK2 signal, functional activation of estrogen receptor alpha in MCF-7 cells. J Biol Chem. 2004;279:19908–15.
Mantzoros C, Petridou E, Dessypris N, et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab. 2004;89:1102–7.
Kang JH, Lee YY, Yu BY, et al. Adiponectin induces growth arrest and apoptosis of MDA-MB-231 breast cancer cell. Arch Pharm Res. 2005;28:1263–9.
Bodmer M, Meier C, Krahenbuhl S, et al. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care. 2010;33:1304–8.
Marshall S (2011) Role of insulin, adipocyte hormones, and nutrient-sensing pathways in regulating fuel metabolism and energy homeostasis: a nutritional perspective of diabetes, obesity, and cancer. Available at http://stke.sciencemag.org/cgi/reprint/sigtrans;2006/346/re7.pdf. Accessed May 2011.
Yang YX, Hennessy S, Lewis JD. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology. 2004;127:1044–50.
Lipscombe LL, Goodwin PJ, Zinman B, et al. Increased prevalence of prior breast cancer in women with newly diagnosed diabetes. Breast Cancer Res Treat. 2006;98:303–9.
Jiralerspong S, Palla SL, Giordano SH, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27:3297–302.
Begg CB, Haile RW, Borg A, et al. Variation of breast cancer risk among BRCA1/2 carriers. JAMA. 2008;299:194–201.
Fabian C. Tamoxifen or raloxifene in postmenopausal women for prevention of breast cancer: a tale of two choices–counterpoint. Cancer Epidemiol Biomarkers Prev. 2007;16:2210–2.
Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–16.
Phoenix KN, Vumbaca F, Claffey KP. Therapeutic metformin/AMPK activation promotes the angiogenic phenotype in the ERalpha negative MDA-MB-435 breast cancer model. Breast Cancer Res Treat. 2009;113:101–11.
Anisimov VN, Berstein LM, Egormin PA, et al. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol. 2005;40:685–93.
Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. The antidiabetic drug metformin suppresses HER2 (erbB-2) oncoprotein over expression via inhibition of the mTOR effector p70S6K1 in human breast carcinoma cells. Cell Cycle. 2009;8:88–96.
Buzzai M, Jones RG, Amaravadi RK, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–52.
Saal LH, Gruvberger-Saal SK, Persson C, et al. Recurrent gross mutations of the PTEN tumor suppressor gene in breast cancers with deficient DSB repair. Nat Genet. 2008;40:102–7.
Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation–AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol. 2006;574:63–71.
Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74.
Holland W, Morrison T, Chang Y, et al. Metformin (Glucophage) inhibits tyrosine phosphatase activity to stimulate the insulin receptor tyrosine kinase. Biochem Pharmacol. 2004;67:2081–91.
Shaw RJ, Lamia KA, Vasquez D, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–6.
Petricoin EF, Espina V, Araujo RP, Liotta LA. Phosphoprotein pathway mapping: AKT/mammalian target of rapamycin activation is negatively associated with childhood rhabdomyosarcoma survival. Cancer Res. 2007;67:3431–340.
Evans JM, Donnelly LA, Emslie-Smith AM, et al. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–5.
Libby G, Donnelly LA, Donnan PT, et al. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–5.
Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–8.
Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–77.
Landman GWD, Kleefstra N, van Hateren KJJ, et al. Metformin associated with lower cancer mortality in type 2 diabetes—ZODIAC-16. Diabetes Care. 2010;33:322–6.
Bodmer M, Meier C, Krähenbühl S, et al. Long-Term Metformin Use Is Associated With Decreased Risk of Breast Cancer. Diabetes Care. 2010;33:1304–8.
Jiralerspong S, Palla SL, Giordano SH, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27:3297–302.
Iliopoulous D, Hirsch HA, Wang G, Struhl K. Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc Natl Acad Sci USA. 2011;108:1397–402.
Oliveras-Ferraros C, Cufi S, Vazquez-Martin A, et al. Micro(mi)RNA expression profile of breast cancer epithelial cells treated with the anti-diabetic drug metformin: induction of the tumor suppressor miRNA let-7a and suppression of the TGF beta-induced oncomiR miRNA-181a. Cell Cycle. 2011;10:1144–51.
Vazquez-Martin A, Oliveras-Ferraros C, Del Barco S, et al. The anti-diabetic drug metformin suppresses self-renewal and proliferation of trastuzumab-resistant tumor-initiating breast cancer stem cells. Breast Cancer Res Treat. 2011;126:355–64.
Disclosure
No conflicts of interest relevant to this article were reported.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Ibarra-Drendall, C., Dietze, E.C. & Seewaldt, V.L. Metabolic Syndrome and Breast Cancer Risk: Is There a Role for Metformin?. Curr Breast Cancer Rep 3, 142–150 (2011). https://doi.org/10.1007/s12609-011-0050-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12609-011-0050-8