Abstract
Objectives
High salt intake results in various harmful effects on human health including hypertension, cardiovascular disease, and reduced bone density. Despite this, there are very few studies in the literature that have investigated the association between sodium intake and osteoarthritis (OA). Therefore, we aimed to explore these associations in a Korean population.
Methods
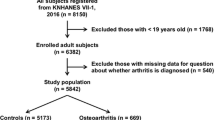
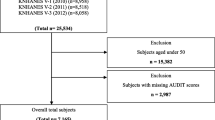
This study used cross-sectional data from adult subjects aged 50–75 years from two consecutive periods of the Korean National Health and Nutrition Examination Survey V–VII (2010–2011 and 2014–2016). The estimated 24-hour urinary sodium excretion (24HUNa) was used as a surrogate marker of salt intake. In the 2010–2011 dataset, knee OA (KOA) was defined as the presence of the radiographic features of OA and knee pain. The association between KOA and salt intake was analysed using univariable and multivariable logistic regression methods. For the sensitivity analysis, the same procedures were conducted on subjects with self-reported OA (SR-OA) with knee pain in the 2010–2011 dataset and any site SR-OA in the 2014–2016 dataset.
Results
Subjects with KOA had significantly lower energy intake, but higher 24HUNa than those without KOA. The restricted cubic spline plots demonstrated a J-shaped distribution between 24HUNa and prevalent KOA. When 24HUNa was stratified into five groups (<2, 2–3, 3–4, 4–5 and ≥5 g/day), subjects with high sodium intake (≥5 g/day) had a higher risk of KOA (odds ratio [OR] = 1.64, 95% confidence interval [CI] 1.03–2.62) compared to the reference group (3–4 g/day) after adjusting for covariates. The sensitivity analysis based on SR-OA with knee pain showed that high sodium intake was also significantly associated with increased prevalence of OA (OR = 1.84, 95% CI 1.10–3.10) compared with the reference group. Regarding SR-OA at any site in the 2014–2016 dataset, estimated 24HUNa showed a significantly positive association with the presence of SR-OA after adjusting for potential confounders.
Conclusions
This nationwide Korean representative study showed a significant association between symptomatic KOA and high sodium intake (≥5 g/day). Avoidance of a diet high in salt might be beneficial as a non-pharmacologic therapy for OA.


Similar content being viewed by others
References
Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB, Goldring SR, Jones G, Teichtahl AJ, Pelletier JP. Osteoarthritis. Nat Rev Dis Primers 2016;2:16072. doi:https://doi.org/10.1038/nrdp.2016.72
Liu-Bryan R, Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat Rev Rheumatol 2015;11(1):35–44. doi:https://doi.org/10.1038/nrrheum.2014.162
Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet 2019;393(10182):1745–1759. doi:https://doi.org/10.1016/s0140-6736(19)30417-9
Safiri S, Kolahi AA, Smith E, Hill C, Bettampadi D, Mansournia MA, Hoy D, Ashrafi-Asgarabad A, Sepidarkish M, Almasi-Hashiani A, Collins G, Kaufman J, Qorbani M, Moradi-Lakeh M, Woolf AD, Guillemin F, March L, Cross M. Global, regional and national burden of osteoarthritis 1990–2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis 2020;79(6):819–828. doi:https://doi.org/10.1136/annrheumdis-2019-216515
Thomas S, Browne H, Mobasheri A, Rayman MP. What is the evidence for a role for diet and nutrition in osteoarthritis? Rheumatology (Oxford) 2018;57(suppl_4):iv61–iv74. doi:https://doi.org/10.1093/rheumatology/key011
Lu B, Driban JB, Xu C, Lapane KL, McAlindon TE, Eaton CB. Dietary Fat Intake and Radiographic Progression of Knee Osteoarthritis: Data From the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2017;69(3):368–375. doi:https://doi.org/10.1002/acr.22952
Dai Z, Niu J, Zhang Y, Jacques P, Felson DT. Dietary intake of fibre and risk of knee osteoarthritis in two US prospective cohorts. Ann Rheum Dis 2017;76(8):1411–1419. doi:https://doi.org/10.1136/annrheumdis-2016-210810
Joseph GB, McCulloch CE, Nevitt MC, Neumann J, Lynch JA, Lane NE, Link TM. Associations Between Vitamins C and D Intake and Cartilage Composition and Knee Joint Morphology Over 4 Years: Data From the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2020;72(9):1239–1247. doi:https://doi.org/10.1002/acr.24021
Misra D, Booth SL, Tolstykh I, Felson DT, Nevitt MC, Lewis CE, Torner J, Neogi T. Vitamin K deficiency is associated with incident knee osteoarthritis. Am J Med 2013;126(3):243–248. doi:https://doi.org/10.1016/j.amjmed.2012.10.011
Arora V, Singh G, I OS, Ma K, Natarajan Anbazhagan A, Votta-Velis EG, Bruce B, Richard R, van Wijnen AJ, Im HJ. Gut-microbiota modulation: The impact of thegut-microbiotaon osteoarthritis. Gene 2021;785:145619. doi:https://doi.org/10.1016/j.gene.2021.145619
Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE, Lim S, Danaei G, Ezzati M, Powles J. Global sodium consumption and death from cardiovascular causes. N Engl J Med 2014;371(7):624–634. doi:https://doi.org/10.1056/NEJMoa1304127
World Health Organization. Guideline: sodium intake for adults and children [Internet]. WHO; 2012. [cited 23 June, 2021]. Available from: https://www.who.int/publications/i/item/9789241504836.
Dahan S, Segal Y, Shoenfeld Y. Dietary factors in rheumatic autoimmune diseases: a recipe for therapy? Nat Rev Rheumatol 2017;13(6):348–358. doi:https://doi.org/10.1038/nrrheum.2017.42
Liu M, Deng M, Luo Q, Dou X, Jia Z. High-Salt Loading Downregulates Nrf2 Expression in a Sodium-Dependent Manner in Renal Collecting Duct Cells. Front Physiol 2019;10:1565. doi:https://doi.org/10.3389/fphys.2019.01565
Kweon S, Kim Y, Jang MJ, Kim Y, Kim K, Choi S, Chun C, Khang YH, Oh K. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol 2014;43(1):69–77. doi:https://doi.org/10.1093/ije/dyt228
Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16(4):494–502. doi:https://doi.org/10.1136/ard.16.4.494
Pereira D, Peleteiro B, Araújo J, Branco J, Santos RA, Ramos E. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthritis Cartilage 2011;19(11):1270–1285. doi:https://doi.org/10.1016/j.joca.2011.08.009
IPAQ Research Committee. International Physical Activity Questionnaire (IPAQ) [Internet]. 2005. [cited 23 June, 2021]. Available from: https://sites.google.com/site/theipaq/.
McLean R, Cameron C, Butcher E, Cook NR, Woodward M, Campbell NRC. Comparison of 24-hour urine and 24-hour diet recall for estimating dietary sodium intake in populations: A systematic review and meta-analysis. J Clin Hypertens (Greenwich) 2019;21(12):1753–1762. doi:https://doi.org/10.1111/jch.13729
Cobb LK, Anderson CA, Elliott P, Hu FB, Liu K, Neaton JD, Whelton PK, Woodward M, Appel LJ, American Heart Association Council on L, Metabolic H. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: a science advisory from the American Heart Association. Circulation 2014;129(10):1173–1186. doi:https://doi.org/10.1161/CIR.0000000000000015
Tanaka T, Okamura T, Miura K, Kadowaki T, Ueshima H, Nakagawa H, Hashimoto T. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens 2002;16(2):97–103. doi:https://doi.org/10.1038/sj.jhh.1001307
Koo HS, Kim YC, Ahn SY, Oh SW, Kim S, Chin HJ, Park JH. Estimating 24-hour urine sodium level with spot urine sodium and creatinine. J Korean Med Sci 2014;29 Suppl 2(Suppl 2):S97–s102. doi:https://doi.org/10.3346/jkms.2014.29.S2.S97
Toft U, Cerqueira C, Andreasen AH, Thuesen BH, Laurberg P, Ovesen L, Perrild H, Jørgensen T. Estimating salt intake in a Caucasian population: can spot urine substitute 24-hour urine samples? Eur J Prev Cardiol 2014;21(10):1300–1307. doi:https://doi.org/10.1177/2047487313485517
Brown IJ, Dyer AR, Chan Q, Cogswell ME, Ueshima H, Stamler J, Elliott P. Estimating 24-hour urinary sodium excretion from casual urinary sodium concentrations in Western populations: the INTERSALT study. Am J Epidemiol 2013;177(11):1180–1192. doi:https://doi.org/10.1093/aje/kwt066
O’Donnell M, Mente A, Rangarajan S, McQueen MJ, O’Leary N, Yin L, Liu X, Swaminathan S, Khatib R, Rosengren A, Ferguson J, Smyth A, Lopez-Jaramillo P, Diaz R, Avezum A, Lanas F, Ismail N, Yusoff K, Dans A, Iqbal R, Szuba A, Mohammadifard N, Oguz A, Yusufali AH, Alhabib KF, Kruger IM, Yusuf R, Chifamba J, Yeates K, Dagenais G, Wielgosz A, Lear SA, Teo K, Yusuf S. Joint association of urinary sodium and potassium excretion with cardiovascular events and mortality: prospective cohort study. BMJ 2019;364:l772. doi:https://doi.org/10.1136/bmj.l772
Mente A, O’Donnell M, Rangarajan S, Dagenais G, Lear S, McQueen M, Diaz R, Avezum A, Lopez-Jaramillo P, Lanas F, Li W, Lu Y, Yi S, Rensheng L, Iqbal R, Mony P, Yusuf R, Yusoff K, Szuba A, Oguz A, Rosengren A, Bahonar A, Yusufali A, Schutte AE, Chifamba J, Mann JF, Anand SS, Teo K, Yusuf S. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies. Lancet 2016;388(10043):465–475. doi:https://doi.org/10.1016/s0140-6736(16)30467-6
Cui A, Li H, Wang D, Zhong J, Chen Y, Lu H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 2020;29–30:100587. doi:https://doi.org/10.1016/j.eclinm.2020.100587
Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ 2013;346:f1326. doi:https://doi.org/10.1136/bmj.f1326
Moosavian SP, Haghighatdoost F, Surkan PJ, Azadbakht L. Salt and obesity: a systematic review and meta-analysis of observational studies. Int J Food Sci Nutr 2017;68(3):265–277. doi:https://doi.org/10.1080/09637486.2016.1239700
Fatahi S, Namazi N, Larijani B, Azadbakht L. The Association of Dietary and Urinary Sodium With Bone Mineral Density and Risk of Osteoporosis: A Systematic Review and Meta-Analysis. J Am Coll Nutr 2018;37(6):522–532. doi:https://doi.org/10.1080/07315724.2018.1431161
Matsunaga M, Lim E, Davis J, Chen JJ. Dietary Quality Associated with Self-Reported Diabetes, Osteoarthritis, and Rheumatoid Arthritis among Younger and Older US Adults: A Cross-Sectional Study Using NHANES 2011:2016. Nutrients 2021;13(2). doi:https://doi.org/10.3390/nu13020545
Kim I, Kim HA, Seo YI, Song YW, Jeong JY, Kim DH. The prevalence of knee osteoarthritis in elderly community residents in Korea. J Korean Med Sci 2010;25(2):293–298. doi:https://doi.org/10.3346/jkms.2010.25.2.293
Cho NH, Kim S, Kim HA, Seo YI. The prevalence and risk factors of knee and hand osteoarthritis in Korea. J Rheum Dis 2007;14(4):354–362. doi:https://doi.org/10.4078/jkra.2007.14.4.354
Alderman MH, Cohen HW. Dietary sodium intake and cardiovascular mortality: controversy resolved? Am J Hypertens 2012;25(7):727–734. doi:https://doi.org/10.1038/ajh.2012.52
Andrianakos AA, Kontelis LK, Karamitsos DG, Aslanidis SI, Georgountzos AI, Kaziolas GO, Pantelidou KV, Vafiadou EV, Dantis PC. Prevalence of symptomatic knee, hand, and hip osteoarthritis in Greece. The ESORDIG study. J Rheumatol 2006;33(12):2507–2513
Fransen M, Bridgett L, March L, Hoy D, Penserga E, Brooks P. The epidemiology of osteoarthritis in Asia. Int J Rheum Dis 2011;14(2):113–121. doi:https://doi.org/10.1111/j.1756-185X.2011.01608.x
Szoeke CE, Dennerstein L, Wluka AE, Guthrie JR, Taffe J, Clark MS, Cicuttini FM. Physician diagnosed arthritis, reported arthritis and radiological non-axial osteoarthritis. Osteoarthritis Cartilage 2008;16(7):846–850. doi:https://doi.org/10.1016/j.joca.2007.12.001
Panoutsopoulou K, Zeggini E. Advances in osteoarthritis genetics. J Med Genet 2013;50(11):715–724. doi:https://doi.org/10.1136/jmedgenet-2013-101754
MacGregor AJ, Li Q, Spector TD, Williams FM. The genetic influence on radiographic osteoarthritis is site specific at the hand, hip and knee. Rheumatology (Oxford) 2009;48(3):277–280. doi:https://doi.org/10.1093/rheumatology/ken475
Tachmazidou I, Hatzikotoulas K, Southam L, Esparza-Gordillo J, Haberland V, Zheng J, Johnson T, Koprulu M, Zengini E, Steinberg J, Wilkinson JM, Bhatnagar S, Hoffman JD, Buchan N, Süveges D, Yerges-Armstrong L, Smith GD, Gaunt TR, Scott RA, McCarthy LC, Zeggini E. Identification of new therapeutic targets for osteoarthritis through genome-wide analyses of UK Biobank data. Nat Genet 2019;51(2):230–236. doi:https://doi.org/10.1038/s41588-018-0327-1
Moe RH, Grotle M, Kjeken I, Hagen KB, Kvien TK, Uhlig T. Disease impact of hand OA compared with hip, knee and generalized disease in specialist rheumatology health care. Rheumatology (Oxford) 2013;52(1):189–196. doi:https://doi.org/10.1093/rheumatology/kes215
Sayre EC, Li LC, Kopec JA, Esdaile JM, Bar S, Cibere J. The effect of disease site (knee, hip, hand, foot, lower back or neck) on employment reduction due to osteoarthritis. PLoS One 2010;5(5):e10470. doi:https://doi.org/10.1371/journal.pone.0010470
Fan A, Oladiran O, Shi XQ, Zhang J. High-salt diet decreases mechanical thresholds in mice that is mediated by a CCR2-dependent mechanism. J Neuroinflammation 2020;17(1):179. doi:https://doi.org/10.1186/s12974-020-01858-6
Raghu H, Lepus CM, Wang Q, Wong HH, Lingampalli N, Oliviero F, Punzi L, Giori NJ, Goodman SB, Chu CR, Sokolove JB, Robinson WH. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann Rheum Dis 2017;76(5):914–922. doi:https://doi.org/10.1136/annrheumdis-2016-210426
Wenstedt EF, Verberk SG, Kroon J, Neele AE, Baardman J, Claessen N, Pasaoglu Ö T, Rademaker E, Schrooten EM, Wouda RD, de Winther MP, Aten J, Vogt L, Van den Bossche J. Salt increases monocyte CCR2 expression and inflammatory responses in humans. JCI Insight 2019;4(21). doi:https://doi.org/10.1172/jci.insight.130508
Wang Y, Teichtahl AJ, Abram F, Hussain SM, Pelletier JP, Cicuttini FM, Martel-Pelletier J. Knee pain as a predictor of structural progression over 4 years: data from the Osteoarthritis Initiative, a prospective cohort study. Arthritis Res Ther 2018;20(1):250. doi:https://doi.org/10.1186/s13075-018-1751-4
Amer M, Woodward M, Appel LJ. Effects of dietary sodium and the DASH diet on the occurrence of headaches: results from randomised multicentre DASH-Sodium clinical trial. BMJ Open 2014;4(12):e006671. doi:https://doi.org/10.1136/bmjopen-2014-006671
Malta DC, Oliveira MM, Andrade S, Caiaffa WT, Souza MFM, Bernal RTI. Factors associated with chronic back pain in adults in Brazil. Rev Saude Publica 2017;51(suppl 1):9s. doi:https://doi.org/10.1590/s1518-8787.2017051000052
Millerand M, Berenbaum F, Jacques C. Danger signals and inflammaging in osteoarthritis. Clin Exp Rheumatol 2019;37 Suppl 120(5):48–56
Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, Sokolove J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol 2016;12(10):580–592. doi:https://doi.org/10.1038/nrrheum.2016.136
Jin X, Beguerie JR, Zhang W, Blizzard L, Otahal P, Jones G, Ding C. Circulating C reactive protein in osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis 2015;74(4):703–710. doi:https://doi.org/10.1136/annrheumdis-2013-204494
Yilmaz R, Akoglu H, Altun B, Yildirim T, Arici M, Erdem Y. Dietary salt intake is related to inflammation and albuminuria in primary hypertensive patients. Eur J Clin Nutr 2012;66(11):1214–1218. doi:https://doi.org/10.1038/ejcn.2012.110
Lee M, Sorn SR, Lee Y, Kang I. Salt Induces Adipogenesis/Lipogenesis and Inflammatory Adipocytokines Secretion in Adipocytes. Int J Mol Sci 2019;20(1). doi:https://doi.org/10.3390/ijms20010160
Kidd B. Mechanisms of pain in osteoarthritis. Hss j 2012;8(1):26–28. doi:https://doi.org/10.1007/s11420-011-9263-7
de Rooij M, van der Leeden M, Heymans MW, Holla JF, Häkkinen A, Lems WF, Roorda LD, Veenhof C, Sanchez-Ramirez DC, de Vet HC, Dekker J. Prognosis of Pain and Physical Functioning in Patients With Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res (Hoboken) 2016;68(4):481–492. doi:https://doi.org/10.1002/acr.22693
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15(12):1833–1840
Veronese N, Trevisan C, De Rui M, Bolzetta F, Maggi S, Zambon S, Musacchio E, Sartori L, Perissinotto E, Crepaldi G, Manzato E, Sergi G. Association of Osteoarthritis With Increased Risk of Cardiovascular Diseases in the Elderly: Findings From the Progetto Veneto Anziano Study Cohort. Arthritis Rheumatol 2016;68(5):1136–1144. doi:https://doi.org/10.1002/art.39564
Wang H, Bai J, He B, Hu X, Liu D. Osteoarthritis and the risk of cardiovascular disease: a meta-analysis of observational studies. Sci Rep 2016;6:39672. doi:https://doi.org/10.1038/srep39672
Veronese N, Stubbs B, Solmi M, Smith TO, Reginster JY, Maggi S. Osteoarthristis Increases the Risk of Cardiovascular Disease: Data from the Osteoarthritis Initiative. J Nutr Health Aging 2018;22(3):371–376. doi:https://doi.org/10.1007/s12603-017-0941-0
Kim HS, Shin JS, Lee J, Lee YJ, Kim MR, Bae YH, Park KB, Lee EJ, Kim JH, Ha IH. Association between Knee Osteoarthritis, Cardiovascular Risk Factors, and the Framingham Risk Score in South Koreans: A Cross-Sectional Study. PLoS One 2016;11(10):e0165325. doi:https://doi.org/10.1371/journal.pone.0165325
Constantino de Campos G, Mundi R, Whittington C, Toutounji MJ, Ngai W, Sheehan B. Osteoarthritis, mobility-related comorbidities and mortality: an overview of meta-analyses. Ther Adv Musculoskelet Dis 2020;12:1759720x20981219. doi:https://doi.org/10.1177/1759720x20981219
GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019;393(10184):1958–1972. doi:https://doi.org/10.1016/s0140-6736(19)30041-8
Leifer VP, Katz JN, Losina E. The burden of OA-health services and economics. Osteoarthritis Cartilage 2021; doi:https://doi.org/10.1016/j.joca.2021.05.007. doi:https://doi.org/10.1016/j.joca.2021.05.007
Hashimoto H, Nomura N, Shoda W, Isobe K, Kikuchi H, Yamamoto K, Fujimaru T, Ando F, Mori T, Okado T, Rai T, Uchida S, Sohara E. Metformin increases urinary sodium excretion by reducing phosphorylation of the sodium-chloride cotransporter. Metabolism 2018;85:23–31. doi:https://doi.org/10.1016/j.metabol.2018.02.009
Liu C, Zhao Q, Zhen Y, Zhai J, Liu G, Zheng M, Ma G, Wang L, Tian L, Ji L, Li L, Duan L, Liu K. Effect of Corticosteroid on Renal Water and Sodium Excretion in Symptomatic Heart Failure: Prednisone for Renal Function Improvement Evaluation Study. J Cardiovasc Pharmacol 2015;66(3):316–322. doi:https://doi.org/10.1097/fjc.0000000000000282
Shah S, Pitt B, Brater DC, Feig PU, Shen W, Khwaja FS, Wilcox CS. Sodium and Fluid Excretion With Torsemide in Healthy Subjects is Limited by the Short Duration of Diuretic Action. J Am Heart Assoc 2017;6(10). doi:https://doi.org/10.1161/jaha.117.006135
Acknowledgement
The authors thank Division of Statistics in Medical Research Collaborating Center at Seoul National University Bundang Hospital for statistical analysis. We would like to thank Editage (https://www.editage.co.kr) for English language editing.
Funding
Funding: This study was supported by the Seoul National University Bundang Hospital Research Fund (grant no. 02-2018-047 to YJH and 21-2021-0013 to YJL).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest: The authors declare that they have no competing interests.
Ethical approval: This study conformed to the ethical guidelines of the 2008 Declaration of Helsinki and was approved by the Institutional Review Board of Seoul National University Bundang Hospital. All participants provided written informed consent prior to being administered the survey.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ha, YJ., Ji, E., Lee, J.H. et al. High Estimated 24-Hour Urinary Sodium Excretion Is Related to Symptomatic Knee Osteoarthritis: A Nationwide Cross-Sectional Population-Based Study. J Nutr Health Aging 26, 581–589 (2022). https://doi.org/10.1007/s12603-022-1804-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-022-1804-x