Abstract
Objectives
Concurrent Training (CT) is described as a combination of resistance training (RT) and endurance training (ET) in a periodized program to maximize all aspects of physical performance. To date, effects of CT order on muscular and cardiorespiratory fitness adaptations are controversial. Owing to the age-related decrement in satellite cells (SC) which are critical for fiber repair, conservation, muscle hypertrophy as well as cardiorespiratory fitness, the present study examined the response of SC related markers to CT order in older sarcopenic men.
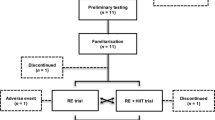
Participants
Thirty older men (age= 64.3 ± 3.5 years) were randomly assigned into one of 3 groups, ET followed by RT (E+R; n=10), RT followed by ET (R+E; n= 10) or a control (C; n=10).
Intervention
The training protocol consisted of 3 exercise sessions per week for 8 weeks. Blood samples were obtained at baseline and 48 hours after the final training session.
Results
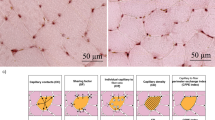
Weight, skeletal muscle mass, lower and upper body power, maximal oxygen consumption (VO2max), Paired Box 7 (Pax7), and Myogenic factor 5 (Myf5) significantly increased, while were percent body fat significantly decreased following E+R and R+E compared to C. Importantly, the improvement in skeletal muscle mass, lower and upper body power, Myf5 and Pax7 in the E+R was significantly greater than the R+E group. Myogenin (Myog) and Paired Box 3 (Pax3) significantly increased (P < 0.01) in both training groups compared to no changes in C.
Conclusion
An 8-week CT intervention improves SC related markers, body composition and enhances power and VO2max in older sarcopenic participants, regardless of the order of RT and ET. However, performing ET before RT may be more effective at enhancing skeletal muscle mass, Myf5 and Pax7, in addition to both lower and upper body power. While both CT programs produced notable physiological and performance benefits, performing ET before RT during CT may provide the greatest therapeutic benefits for aging individuals.



Similar content being viewed by others
References
Bagheri, R., et al., Effects of upper-body, lower-body, or combined resistance training on the ratio of follistatin and myostatin in middle-aged men. European journal of applied physiology, 2019: p. 1–11.
Bagheri, R., et al., The effects of concurrent training order on body composition and serum concentrations of follistatin, myostatin and GDF11 in sarcopenic elderly men. Experimental Gerontology, 2020. 133: p. 110869.
Larsson, L., B. Sjödin, and J. Karlsson, Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiologica Scandinavica, 1978. 103(1): p. 31–39.
Dreyer, H.C., et al., Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle & Nerve: Official Journal of the American Association of Electrodiagnostic Medicine, 2006. 33(2): p. 242–253.
Snijders, T., L.B. Verdijk, and L.J. van Loon, The impact of sarcopenia and exercise training on skeletal muscle satellite cells. Ageing research reviews, 2009. 8(4): p. 328–338.
White, J. and G. Smythe, Growth Factors and Cytokines in Skeletal Muscle Development, Growth, Regeneration and Disease. Vol. 900. 2016: Springer.
Renault, V., et al., Regenerative potential of human skeletal muscle during aging. Aging cell, 2002. 1(2): p. 132–139.
Kadi, F., et al., Satellite cells and myonuclei in young and elderly women and men. Muscle & Nerve: Official Journal of the American Association of Electrodiagnostic Medicine, 2004. 29(1): p. 120–127.
Allen, D.L., R.R. Roy, and V.R. Edgerton, Myonuclear domains in muscle adaptation and disease. Muscle & nerve, 1999. 22(10): p. 1350–1360.
Olsen, S., et al., Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training. The Journal of physiology, 2006. 573(2): p. 525–534.
Verdijk, L.B., et al., Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 2009. 64(3): p. 332–339.
Mackey, A., et al., Enhanced satellite cell proliferation with resistance training in elderly men and women. Scandinavian journal of medicine & science in sports, 2007. 17(1): p. 34–42.
Marcinik, E.J., et al., Effects of strength training on lactate threshold and endurance performance. Medicine and science in sports and exercise, 1991. 23(6): p. 739–743.
Ross, R., et al., Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation, 2016. 134(24): p. e653–e699.
de Souza, E.O., et al., Effects of concurrent strength and endurance training on genes related to myostatin signaling pathway and muscle fiber responses. The Journal of Strength & Conditioning Research, 2014. 28(11): p. 3215–3223.
Fyfe, J.J., D.J. Bishop, and N.K. Stepto, Interference between concurrent resistance and endurance exercise: molecular bases and the role of individual training variables. Sports medicine, 2014. 44(6): p. 743–762.
Coffey, V.G. and J.A. Hawley, Concurrent exercise training: do opposites distract? The Journal of physiology, 2017. 595(9): p. 2883–2896.
Wilson, J.M., et al., Concurrent training: a meta-analysis examining interference of aerobic and resistance exercises. The Journal of Strength & Conditioning Research, 2012. 26(8): p. 2293–2307.
Cadore, E.L., et al., Neuromuscular adaptations to concurrent training in the elderly: effects of intrasession exercise sequence. Age, 2013. 35(3): p. 891–903.
Verney, J., et al., Effects of combined lower body endurance and upper body resistance training on the satellite cell pool in elderly subjects. Muscle & Nerve: Official Journal of the American Association of Electrodiagnostic Medicine, 2008. 38(3): p. 1147–1154.
Snijders, T., et al., Prolonged exercise training improves the acute type II muscle fibre satellite cell response in healthy older men. The Journal of physiology, 2019. 597(1): p. 105–119.
Moore, D.R., et al., Low-load resistance exercise during inactivity is associated with greater fibre area and satellite cell expression in older skeletal muscle. Journal of cachexia, sarcopenia and muscle, 2018. 9(4): p. 747–754.
Pugh, J.K., et al., Satellite cell response to concurrent resistance exercise and high-intensity interval training in sedentary, overweight/obese, middle-aged individuals. European journal of applied physiology, 2018. 118(2): p. 225–238.
Babcock, L., et al., Concurrent aerobic exercise interferes with the satellite cell response to acute resistance exercise. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 2012. 302(12): p. R1458–R1465.
Bagheri, R., et al., Effects of green tea extract supplementation and endurance training on irisin, pro-inflammatory cytokines, and adiponectin concentrations in overweight middle-aged men. European Journal of Applied Physiology, 2020: p. 1–9.
Murach, K.A. and J.R. Bagley, Skeletal muscle hypertrophy with concurrent exercise training: contrary evidence for an interference effect. Sports medicine, 2016. 46(8): p. 1029–1039.
Fatouros, I., et al., Strength training and detraining effects on muscular strength, anaerobic power, and mobility of inactive older men are intensity dependent. British journal of sports medicine, 2005. 39(10): p. 776–780.
Miszko, T.A., et al., Effect of strength and power training on physical function in community-dwelling older adults. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 2003. 58(2): p. M171–M175.
Medicine, A.C.O.S., ACSM’s guidelines for exercise testing and prescription. 2013: Lippincott Williams & Wilkins.
Church, D.D., et al., Comparison of high-intensity vs. high-volume resistance training on the BDNF response to exercise. Journal of Applied Physiology, 2016. 121(1): p. 123–128.
Cadore, E., et al., Physiological effects of concurrent training in elderly men. International journal of sports medicine, 2010. 31(10): p. 689–697.
Zdzieblik, D., et al., Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: a randomised controlled trial. British Journal of Nutrition, 2015. 114(8): p. 1237–1245.
Jacobs, P.L., NSCA’s Essentials of Training Special Populations. 2017: Human Kinetics.
Huang, G., et al., Dose-response relationship of cardiorespiratory fitness adaptation to controlled endurance training in sedentary older adults. European journal of preventive cardiology, 2016. 23(5): p. 518–529.
Sun, X., et al., Effects of chronic endurance exercise training on serum 25 (OH) D concentrations in elderly Japanese men. Endocrine, 2018. 59(2): p. 330–337.
Bagheri, R., et al., Does Green Tea Extract Enhance the Anti-inflammatory Effects of Exercise on Fat Loss? British journal of clinical pharmacology, 2019.
Thomas, D.T., K.A. Erdman, and L.M. Burke, Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: nutrition and athletic performance. Journal of the Academy of Nutrition and Dietetics, 2016. 116(3): p. 501–528.
Kim, J.-S., et al., Resting and load-induced levels of myogenic gene transcripts differ between older adults with demonstrable sarcopenia and young men and women. Journal of applied physiology, 2005. 99(6): p. 2149–2158.
Kosek, D.J., et al., Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. Journal of applied physiology, 2006. 101(2): p. 531–544.
Nederveen, J., et al., The effect of exercise mode on the acute response of satellite cells in old men. Acta Physiologica, 2015. 215(4): p. 177–190.
Bazgir, B., et al., Satellite cells contribution to exercise mediated muscle hypertrophy and repair. Cell Journal (Yakhteh), 2017. 18(4): p. 473.
Sabourin, L.A. and M.A. Rudnicki, The molecular regulation of myogenesis. Clinical genetics, 2000. 57(1): p. 16–25.
McKay, B.R., et al., Co-expression of IGF-1 family members with myogenic regulatory factors following acute damaging muscle-lengthening contractions in humans. The Journal of physiology, 2008. 586(22): p. 5549–5560.
Wang, Y.X. and M.A. Rudnicki, Satellite cells, the engines of muscle repair. Nature reviews Molecular cell biology, 2012. 13(2): p. 127.
Abreu, P., et al., Satellite cell activation induced by aerobic muscle adaptation in response to endurance exercise in humans and rodents. Life sciences, 2017. 170: p. 33–40.
Macaluso, F., et al., Satellite cell count, VO2max, and p38 MAPK in inactive to moderately active young men. Scandinavian journal of medicine & science in sports, 2012. 22(4): p. e38–e44.
Schwingshackl, L., et al., Impact of different training modalities on anthropometric and metabolic characteristics in overweight/obese subjects: a systematic review and network meta-analysis. PloS one, 2013. 8(12): p. e82853.
Cadore, E.L., et al., Strength prior to endurance intra-session exercise sequence optimizes neuromuscular and cardiovascular gains in elderly men. Experimental gerontology, 2012. 47(2): p. 164–169.
Banitalebi, E. and H.B. Baghanari, Effect of sequence order of combined training (resistance and endurance) on strength, aerobic capacity, and body composition in older women. Middle East Journal of Rehabilitation and Health, 2015. 2(2).
Sheikholeslami-Vatani, D., et al., The effect of concurrent training order on hormonal responses and body composition in obese men. Science & Sports, 2015. 30(6): p. 335–341.
Roth, S., et al., Skeletal muscle satellite cell characteristics in young and older men and women after heavy resistance strength training. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 2001. 56(6): p. B240–B247.
Manini, T.M., Energy expenditure and aging. Ageing research reviews, 2010. 9(1): p. 1–11.
Krems, C., et al., Lower resting metabolic rate in the elderly may not be entirely due to changes in body composition. European journal of clinical nutrition, 2005. 59(2): p. 255.
Enright, K., et al., The effect of concurrent training organisation in youth elite soccer players. European journal of applied physiology, 2015. 115(11): p. 2367–2381.
Davitt, P.M., et al., The effects of a combined resistance training and endurance exercise program in inactive college female subjects: does order matter? The Journal of Strength & Conditioning Research, 2014. 28(7): p. 1937–1945.
Schoenfeld, B.J., The mechanisms of muscle hypertrophy and their application to resistance training. The Journal of Strength & Conditioning Research, 2010. 24(10): p. 2857–2872.
Laplante, M. and D.M. Sabatini, mTOR signaling at a glance. Journal of cell science, 2009. 122(20): p. 3589–3594.
Kapahi, P., et al., With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell metabolism, 2010. 11(6): p. 453–465.
Mounier, R., et al., Antagonistic control of muscle cell size by AMPK and mTORC1. Cell Cycle, 2011. 10(16): p. 2640–2646.
Ogasawara, R., et al., The order of concurrent endurance and resistance exercise modifies mTOR signaling and protein synthesis in rat skeletal muscle. American Journal of Physiology-Endocrinology and Metabolism, 2014. 306(10): p. E1155–E1162.
Apró, W., et al., Resistance exercise-induced S6K1 kinase activity is not inhibited in human skeletal muscle despite prior activation of AMPK by high-intensity interval cycling. American Journal of Physiology-Endocrinology and Metabolism, 2015. 308(6): p. E470–E481.
Chtara, M., et al., Effect of concurrent endurance and circuit resistance training sequence on muscular strength and power development. The Journal of Strength & Conditioning Research, 2008. 22(4): p. 1037–1045.
Wilhelm, E.N., et al., Concurrent strength and endurance training exercise sequence does not affect neuromuscular adaptations in older men. Experimental gerontology, 2014. 60: p. 207–214.
Smith, L.L., Causes of delayed onset muscle soreness and the impact on athletic performance: a review. The Journal of Strength & Conditioning Research, 1992. 6(3): p. 135–141.
Davis, W.J., et al., Elimination of delayed-onset muscle soreness by pre-resistance cardioacceleration before each set. The Journal of Strength & Conditioning Research, 2008. 22(1): p. 212–225.
Schumann, M., et al., Fitness and lean mass increases during combined training independent of loading order. Medicine and science in sports and exercise, 2014. 46(9).
Hawkins, S.A. and R.A. Wiswell, Rate and mechanism of maximal oxygen consumption decline with aging. Sports medicine, 2003. 33(12): p. 877–888.
Blair, S.N., et al., Physical fitness and all-cause mortality: a prospective study of healthy men and women. Jama, 1989. 262(17): p. 2395–2401.
Ahtiainen, J.P., et al., Muscle hypertrophy, hormonal adaptations and strength development during strength training in strength-trained and untrained men. European journal of applied physiology, 2003. 89(6): p. 555–563.
Kraemer, W.J., et al., Hormonal and growth factor responses to heavy resistance exercise protocols. Journal of Applied Physiology, 1990. 69(4): p. 1442–1450.
Acknowledgements
The authors wish to thank all the participants in this research project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest: The authors declare no conflict of interest.
Disclosure statement: Babak Hooshmand Moghadam has nothing to disclose; Reza Bagheri has nothing to disclose; Damoon Ashtary-Larky has nothing to disclose; Grant M. Tinsley has nothing to disclose; Mozhgan Eskandari has nothing to disclose; Julien S Baker has nothing to disclose; Bizhan Hooshmand Moghadam has nothing to disclose; Richard B. Kreider has nothing to disclose; Alexei Wong has nothing to disclose.
Financial Support: This study was supported by Faculty of sports sciences of Tehran University.
Ethical standard: This study was reviewed and approved by the institutional review board of Tehran University.
Rights and permissions
About this article
Cite this article
Moghadam, B.H., Bagheri, R., Ashtary-Larky, D. et al. The Effects of Concurrent Training Order on Satellite Cell-Related Markers, Body Composition, Muscular and Cardiorespiratory Fitness in Older Men with Sarcopenia. J Nutr Health Aging 24, 796–804 (2020). https://doi.org/10.1007/s12603-020-1431-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-020-1431-3