Abstract
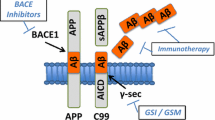
Alzheimer’s disease (AD) is an age-related neurodegenerative disease with a global prevalence estimated at 26.55 million in 2006. During the past decades, several agents have been approved that enhance cognition of AD patients. However, the effectiveness of these treatments are limited or controversial and they do not modify disease progression. Recent advances in understanding AD pathogenesis have led to the development of numerous compounds that might modify the disease process. AD is mainly characterized neuropathologically by the presence of two kinds of protein aggregates: extracellular plaques of Abeta-peptide and intracellular neurofibrillary tangles. Abeta and tau could interfere in an original way contributing to a cascade of events leading to neuronal death and transmitter deficits. Investigation for novel therapeutic approaches targeting the presumed underlying pathogenic mechanisms is major focus of research. Antiamyloid agents targeting production, accumulation, clearance, or toxicity associated with Abeta peptide, are some approaches under investigation to limit extracellular plaques of Abeta-peptide accumulation. We can state as an example: Abeta passive and active immunization, secretases modulation, Abeta degradation enhancement, or antiaggregation and antifibrillization agents. Tau-related therapies are also under clinical investigation but few compounds are available. Another alternative approach under development is neuroprotective agents such as antioxidants, anti-inflammatory drugs, compounds acting against glutamate mediated neurotoxicity. Neurorestorative approaches through neurotrophin or cell therapy also represent a minor avenue in AD research. Finally, statins, receptor for advanced glycation end products inhibitors, thiazolidinediones, insulin, and hormonal therapies are some other ways of research for a therapeutic approach of Alzheimer’s disease. Taking into account AD complexity, it becomes clear that polypharmacology with drugs targeting different sites could be the future treatment approach and a majority of the recent drugs under evaluation seems to act on multiple targets. This article exposes general classes of disease-modifying therapies under investigation.
Similar content being viewed by others
References
Brookmeyer R, Johnson E, Ziegler G, et al. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement 2007;3:186–191.
Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database of Systematic Reviews 2006;1:CD005593.
McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database of Systematic Reviews 2006;2: CD003154.
Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med 2008;148:379–397.
Iwatsubo T, Odaka A, Suzuki N, et al. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43). Neuron1994;13:45–53.
Hardy J. Has the amyloid cascade hypothesis for Alzheimer’s disease been proved? Curr Alzheimer Res 2006;3:71–73.
Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 2002;297:353–356.
Ghiso J, Frangione B. Cerebral amyloidosis, amyloid angiopathy, and their relationship to stroke and dementia. J Alzheimers Dis 2001;3:65–73.
Iqbal K, Grundke-Iqbal I. Discoveries of tau, abnormally hyperphosphorylated tau and others of neurofibrillary degeneration: a personal historical perspective. J Alzheimers Dis. 2006;9:219–242.
Iqbal K, Grundke-Iqbal I. Alzheimer neurofibrillary degeneration: significance, etiopathogenesis, therapeutics and prevention. J Cell Mol Med 2008; 12:38–55.
Iqbal K, Grundke-Iqbal I. Pharmacological approaches of neurofibrillary degeneration. Curr Alzheimer Res 2005; 2:335–341.
Zheng WH, Bastianetto S, Mennicken F, et al. Amyloid beta peptide induces tau phosphorylation and loss of cholinergic neurons in rat primary septal cultures. Neuroscience 2002;115:201–211.
Arriagada PV, Growdon JH, Hedley-Whyte ET, et al. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 1992; 42: 631–639.
Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx 2004;1:213–225.
Selkoe DJ, Schenk D. Alzheimer’s disease: molecular understanding predicts amyloid-based therapeutics. Annu Rev Pharmacol Toxicol 2003;43:545–584.
Schenk D, Barbour R, Dunn W, et al. Immunization with amyloid-Beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 1999;400:173–177.
Morgan D, Diamond DM, Gottschall PE, et al. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature 2000;408:982–985.
Lavie V, Becker M, Cohen-Kupiec R, et al. EFRH-phage immunization of Alzheimer’s disease animal model improves behavioral performance in Morris water maze trials. J Mol Neurosci 2004;24:105–113.
Orgogozo JM, Gilman S, Dartigues JF, et al. Subacute meningoencephalitis in a subset of patients with AD after ABeta42 immunization. Neurology 2003;61:46–54.
Hock C, Konietzko U, Streffer JR, et al. Antibodies against Beta-amyloid slow cognitive decline in Alzheimer’s disease. Neuron 2003;38:547–554.
Gilman S, Koller M, Black RS, et al. Clinical effects of ABeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 2005;64:1553–1562.
Woodhouse A, Dickson TC, Vickers JC. Vaccination strategies for Alzheimer’s disease: A new hope? Drugs Aging 2007;24:107–119.
Pride M, Seubert P, Grundman M, et al. Progress in the active immunotherapeutic approach to Alzheimer’s disease: clinical investigations into AN1792-associated meningoencephalitis. Neurodegener Dis 2008;5:194–196.
Bard F, Cannon C, Barbour R, et al. Peripherally administered antibodies against amyloid Beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med 2000;6:916–919.
DeMattos RB, Bales KR, Cummins DJ, et al. Peripheral anti-A Beta antibody alters CNS and plasma A Beta clearance and decreases brain A Beta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 2001;98:8850–8855.
Goni F, Sigurdsson EM. New directions towards safer and effective vaccines for Alzheimer’s disease. Curr Opin Mol Ther 2005;7:17–23.
Racke MM, Boone LI, Hepburn DL, et al. Exacerbation of cerebral amyloid angiopathy associated microhemorrhage in amyloid precursor protein transgenic mice by immunotherapy is dependent on antibody recognition of deposited forms of amyloid Beta. J Neurosci 2005;25:629–636.
Rakover I, Arbel M, Solomon B. Immunotherapy against APP beta-secretase cleavage site improves cognitive function and reduces neuroinflammation in Tg2576 mice without a significant effect on brain abeta levels. Neurodegener Dis 2007;4:392–402.
Dodel RC, Du Y, Depboylu C, et al. Intravenous immunoglobulins containing antibodies against Beta-amyloid for the treatment of Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2004; 75:1472–1474.
Lichtenthaler SF, Haass C. Amyloid at the cutting edge: activation of alpha-secretase prevents amyloidogenesis in an Alzheimer disease mouse model. J Clin Invest 2004;113:1384–1387.
Luo Y, Bolon B, Kahn S, et al. Mice deficient in BACE1, the Alzheimer’s Betasecretase, have normal phenotype and abolished Beta-amyloid generation. Nat Neurosci 2001;4:231–232.
Hussain I, Hawkins J, Harrison D, et al. Oral administration of a potent and selective non-peptidic BACE-1 inhibitor decreases beta-cleavage of amyloid precursor protein and amyloid-beta production in vivo. J Neurochem 2007;100:802–809.
Rajendran L, Schneider A, Schlechtingen G, et al. Efficient inhibition of the Alzheimer’s disease beta-secretase by membrane targeting. Science 2008;320:520–523.
Comery TA, Martone RL, Aschmies S, et al. Acute gamma-secretase inhibition improves contextual fear conditioning in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci 2005;25:8898–8902.
Dovey HF, John V, Anderson JP, et al. Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J Neurochem 2001;76:173–181.
Hartmann D, Tournoy J, Saftig P, et al. Implication of APP secretases in notch signaling. J Mol Neurosci 2001;17:171–181.
Barten DM, Meredith JE Jr, Zaczek R, et al. Gamma-secretase inhibitors for Alzheimer’s disease: balancing efficacy and toxicity. Drugs R D 2006;7:87–97.
Wong GT, Manfra D, Poulet FM, et al. Chronic treatment with the gamma-secretase inhibitor LY-411,575 inhibits beta-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem 2004;279:12876–12882.
Siemers E, Skinner M, Dean RA, et al. Safety, tolerability, and changes in amyloid beta concentrations after administration of a gamma-secretase inhibitor in volunteers. Clin Neuropharmacol 2005;28:126–132
Siemers ER, Dean RA, Friedrich S, et al. Safety, Tolerability, and Effects on Plasma and Cerebrospinal Fluid Amyloid-beta After Inhibition of gamma-Secretase. Clin Neuropharmacol 2007;30:317–325.
Siemers ER, Quinn JF, Kaye J, et al. Effects of a gamma-secretase inhibitor in a randomized study of patients with Alzheimer disease. Neurology 2006;66:602–604.
Frick G., Raje S., Wan H., et al. P4-366: GSI-953, a potent and selective gammasecretase inhibitor: Modulation of beta-amyloid peptides and plasma and cerebrospinal fluid pharmacokinetic/pharmacodynamic relationships in humans. Alzheimer’s and Dementia 2008;4:T781.
Sun MK, Alkon DL. Protein kinase C isozymes: memory therapeutic potential. Curr Drug Targets CNS Neurol Disord 2005;4:541–552.
Etcheberrigaray R, Tan M, Dewachter I, et al. Therapeutic effects of PKC activators in Alzheimer’s disease transgenic mice. Proc Natl Acad Sci U S A 2004;101:11141–11146.
Wilcock GK, Black SE, Hendrix SB, et al. Efficacy and safety of tarenflurbil in mild to moderate Alzheimer’s disease: a randomised phase II trial. Lancet Neurol 2008;7:483–493.
Nalivaeva NN, Fisk LR, Belyaev ND, et al. Amyloid-degrading enzymes as therapeutic targets in Alzheimer’s disease. Curr Alzheimer Res 2008;5:212–224.
Leissring MA, Farris W, Chang AY, et al. Enhanced proteolysis of Beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron 2003;40:1087–1093.
Yasojima K, McGeer EG, McGeer PL. Relationship between beta amyloid peptide generating molecules and neprilysin in Alzheimer disease and normal brain. Brain Res 2001;919:115–121
Marr RA, Rockenstein E, Mukherjee A, et al. Neprilysin gene transfer reduces human amyloid pathology in transgenic mice. J Neurosci 2003;23:1992–1996.
Saito T, Iwata N, Tsubuki S, et al. Somatostatin regulates brain amyloid Beta peptide ABeta42 through modulation of proteolytic degradation. Nat Med 2005;11:434–439.
Iwata N, Higuchi M, Saido TC. Metabolism of amyloid-beta peptide and Alzheimer’s disease. Pharmacol Ther 2005;108:129–148.
Tokita K, Inoue T, Yamazaki S, et al. FK962, a novel enhancer of somatostatin release, exerts cognitive-enhancing actions in rats. Eur J Pharmacol 2005;527:111–120.
Jacobsen JS, Comery TA, Martone RL, et al. Enhanced clearance of Abeta in brain by sustaining the plasmin proteolysis cascade. Proc Natl Acad Sci U S A 2008;105:8754–8759.
Aisen PS. The development of anti-amyloid therapy for Alzheimer’s disease: from secretase modulators to polymerisation inhibitors. CNS Drugs 2005;19:989–996.
Gervais F, Paquette J, Morissette C, et al. Targeting soluble Abeta peptide with Tramiprosate for the treatment of brain amyloidosis. Neurobiol Aging 2007;28:537–547.
Aisen PS, Saumier D, Briand R, et al. A Phase II study targeting amyloid-beta with 3APS in mild-to-moderate Alzheimer disease. Neurology 2006;67:1757–1763.
Wright TM. Tramiprosate. Drugs Today (Barc) 2006;42:291–298
Finefrock AE, Bush AI, Doraiswamy PM. Current status of metals as therapeutic targets in Alzheimer’s disease. J Am Geriatr Soc 2003;51:1143–1148
Cuajungco MP, Frederickson CJ, Bush AI. Amyloid-beta metal interaction and metal chelation. Subcell Biochem 2005;38:235–254.
Dedeoglu A, Cormier K, Payton S, et al. Preliminary studies of a novel bifunctional metal chelator targeting Alzheimer’s amyloidogenesis. Exp Gerontol 2004;39:1641–1649.
Lee JY, Friedman JE, Angel I, et al. The lipophilic metal chelator DP-109 reduces amyloid pathology in brains of human beta-amyloid precursor protein transgenic mice. Neurobiol Aging 2004;25:1315–1321
Ritchie CW, Bush AI, Mackinnon A, et al. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: a pilot phase 2 clinical trial. Arch Neurol 2003;60:1685–1691.
Sampson E, Jenagaratnam L, McShane R. Metal protein attenuating compounds for the treatment of Alzheimer’s disease. Cochrane Database Syst Rev 2008;1:CD005380.
Liu G, Garrett MR, Men P, et al. Nanoparticle and other metal chelation therapeutics in Alzheimer disease. Biochim Biophys Acta 2005;1741:246–252.
Lannfelt L, Blennow K, Zetterberg H, et al. Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer’s disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol 2008;7:779–786
Jick H, Zornberg GL, Jick SS, et al. Statins and the risk of dementia. Lancet 2000;356:1627–1631.
Canevari L, Clark JB. Alzheimer’s disease and cholesterol: the fat connection. Neurochem Res 2007;32:739–750.
Cordy JM, Hooper NM, Turner AJ. The involvement of lipid rafts in Alzheimer’s disease. Mol Membr Biol 2006;23:111–122
Sparks DL, Sabbagh MN, Connor DJ, et al. Atorvastatin for the treatment of mild to moderate Alzheimer disease: preliminary results. Arch Neurol 2005;62:753–757.
Simons M, Schwärzler F, Lütjohann D, et al. Treatment with simvastatin in normocholesterolemic patients with Alzheimer’s disease: A 26-week randomized, placebo-controlled, double-blind trial. Ann Neurol 2002;52:346–350.
Jones RW, Kivipelto M, Feldman H, et al. The Atorvastatin/Donepezil in Alzheimer’s Disease Study (LEADe): design and baseline characteristics. Alzheimers Dement 2008;4:145–153.
Stuchbury G, Münch G. Alzheimer’s associated inflammation, potential drug targets and future therapies. J Neural Transm 2005;112:429–453.
Lue LF, Walker DG, Brachova L, et al. Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer’s disease: identification of a cellular activation mechanism. Exp Neurol 2001;171:29–45.
Onyango IG, Tuttle JB, Bennett JP Jr. Altered intracellular signaling and reduced viability of Alzheimer’s disease neuronal cybrids is reproduced by beta-amyloid peptide acting through receptor for advanced glycation end products (RAGE). Mol Cell Neurosci 2005;29:333–343.
Maczurek A, Shanmugam K, Münch G. Inflammation and the redox-sensitive AGERAGE pathway as a therapeutic target in Alzheimer’s disease. Ann N Y Acad Sci 2008;1126:147–151
Drewes G. MARKing tau for tangles and toxicity. Trends Biochem Sci 2004;29:548–555.
Munoz L, Ranaivo HR, Roy SM, et al. A novel p38 alpha MAPK inhibitor suppresses brain proinflammatory cytokine up-regulation and attenuates synaptic dysfunction and behavioral deficits in an Alzheimer’s disease mouse model. J Neuroinflammation 2007;4:21.
Phiel CJ, Wilson CA, Lee VM, et al. GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature 2003;423:435–439.
Huang HC, Klein PS. Multiple roles for glycogen synthase kinase-3 as a drug target in Alzheimer’s disease. Curr Drug Targets. 2006 Nov;7(11):1389–1397
Zhao WQ, Feng C, Alkon DL. Impairment of phosphatase 2A contributes to the prolonged MAP kinase phosphorylation in Alzheimer’s disease fibroblasts. Neurobiol Dis 2003;14:458–469.
Tanimukai H, Grundke-Iqbal I, Iqbal K. Up-regulation of inhibitors of protein phosphatase-2A in Alzheimer’s disease. Am J Pathol 2005;166:1761–1771
Wang JZ, Grundke-Iqbal I, Iqbal K. Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur J Neurosci 2007;25:59–68
Wei Q, Holzer M, Brueckner MK, et al. Dephosphorylation of tau protein by calcineurin triturated into neural living cells. Cell Mol Neurobiol 2002;22:13–24
Lian Q, Ladner CJ, Magnuson D, et al. Selective changes of calcineurin (protein phosphatase 2B) activity in Alzheimer’s disease cerebral cortex. Exp Neurol 2001;167:158–165.
Hong M, Chen DC, Klein PS, et al. Lithium reduces tau phosphorylation by inhibition of glycogen synthase kinase-3. J Biol Chem 1997;272:25326–25332.
Rametti A, Esclaire F, Yardin C, et al. Lithium down-regulates tau in cultured cortical neurons: a possible mechanism of neuroprotection. Neurosci Lett 2008;434:93–98.
Gómez-Ramos A, Domínguez J, Zafra D, et al. Inhibition of GSK3 dependent tau phosphorylation by metals. Curr Alzheimer Res 2006;3:123–127
Rockenstein E, Torrance M, Adame A, et al. Neuroprotective effects of regulators of the glycogen synthase kinase-3beta signaling pathway in a transgenic model of Alzheimer’s disease are associated with reduced amyloid precursor protein phosphorylation. J Neurosci 2007;27:1981–1991
Caccamo A, Oddo S, Tran LX, et al. Lithium reduces tau phosphorylation but not A beta or working memory deficits in a transgenic model with both plaques and tangles. Am J Pathol 2007;170:1669–1675
Hampel H, Ewers M, Bürger K, et al. Lithium trial in Alzheimer’s disease: A randomized, single-blinded, placebo-controlled, parallel group multicentre 10-week study. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 2008;4:T782.
Deiana S, Harrington CR, Wischik CM, et al. Methylthioninium chloride reverses cognitive deficits induced by scopolamine: comparison with rivastigmine. Psychopharmacology (Berl) 2008; epub ahead of print.
Nunomura A, Castellani RJ, Zhu X, et al. Involvement of oxidative stress in Alzheimer disease. J Neuropathol Exp Neurol 2006;65:631–641.
Markesbery WR, Carney JM. Oxidative alterations in Alzheimer’s disease. Brain Pathol 1999; 9:133–146.
Keller JN, Schmitt FA, Scheff SW, et al. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology 2005;64:1152–1156.
Nunomura A, Perry G, Aliev G, et al. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol 2001;60:759–767.
Praticò D, Uryu K, Leight S, et al. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci 2001;21:4183–4187.
Engelhart MJ, Geerlings MI, Ruitenberg A, et al. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA 2002;287:3223–3229.
Lewis JM. Vitamin A and Alzheimer’s disease. Neuroepidemiology 1992;11:163–168.
Zandi PP, Anthony JC, Khachaturian AS, et al. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol 2004;61:82–88.
Masaki KH, Losonczy KG, Izmirlian G, et al. Association of vitamin E and C supplement use with cognitive function and dementia in elderly men. Neurology 2000;54:1265–1272.
Laurin D, Masaki KH, Foley DJ, et al. Midlife dietary intake of antioxidants and risk of late-life incident dementia: the Honolulu-Asia Aging Study. Am J Epidemiol 2004;159:959–967.
Wadsworth TL, Bishop JA, Pappu AS, et al. Evaluation of coenzyme Q as an antioxidant strategy for Alzheimer’s disease. J Alzheimers Dis 2008;14:225–234
Young AJ, Johnson S, Steffens DC, et al. Coenzyme Q10: a review of its promise as a neuroprotectant. CNS Spectr 2007;12:62–68.
Kalmijn S, van Boxtel MP, Ocké M, et al. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology 2004;62:275–280.
Morris MC, Evans DA, Bienias JL, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol 2003;60:940–946.
Sano M, Ernesto C, Thomas RG, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. N Engl J Med 1997;336:1216–1222.
Miller ER 3rd, Pastor-Barruiuso R, Dalal D, et al. Meta analysis: High dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005;142:37–46.
Lonn E, Bosch J, Yusuf S, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: A randomized controlled trial. JAMA 2005;293:1338–1347.
Isaac M, Quinn R, Tabet N. Vitamin E for Alzheimer’s disease and mild cognitive impairment. Cochrane Database of Systematic Reviews 2000;4:CD002854.
Lee DH, Folsom AR, Harnack L, et al. Does supplemental vitamin C increase cardiovascular disease risk in women with diabetes? Am J Clin Nutr 2004;80:1194–1200.
Boothby LA, Doering PL. Vitamin C and vitamin E for Alzheimer’s disease. Ann Pharmacother 2005;39:2073–2080.
Barberger-Gateau P, Raffaitin C, Letenneur L, et al. Dietary patterns and risk of dementia: the Three-City cohort study. Neurology 2007;69:1921–1930.
Orr SK, Bazinet RP. The emerging role of docosahexaenoic acid in neuroinflammation. Curr Opin Investig Drugs 2008;9:735–743.
Freund-Levi Y, Eriksdotter-Jönhagen M, Cederholm T, et al. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol 2006;63:1402–1408
Holmquist L, Stuchbury G, Berbaum K, et al. Lipoic acid as a novel treatment for Alzheimer’s disease and related dementias. Pharmacol Ther 2007;113:154–164.
Lovell MA, Xie C, Xiong S, et al. Protection against amyloid beta peptide and iron/hydrogen peroxide toxicity by alpha lipoic acid. J Alzheimers Dis. 2003;5:229–239
Moreira PI, Harris PL, Zhu X, et al. Lipoic acid and N-acetyl cysteine decrease mitochondrial-related oxidative stress in Alzheimer disease patient fibroblasts. J Alzheimers Dis 2007;12:195–206.
Maczurek A, Hager K, Kenklies M, et al. Lipoic acid as an anti-inflammatory and neuroprotective treatment for Alzheimer’s disease. Adv Drug Deliv Rev 2008;60:1463–1470.
Hager K, Kenklies M, McAfoose J, et al. Alpha-lipoic acid as a new treatment option for Alzheimer’s disease—a 48 months follow-up analysis. J Neural Transm Suppl 2007;72:189–193.
Ramassamy C, Longpré F, Christen Y. Ginkgo biloba extract (EGb 761) in Alzheimer’s disease: is there any evidence? Curr Alzheimer Res 2007;4:253–262.
Luo Y, Smith JV, Paramasivam V, et al. Inhibition of amyloid-beta aggregation and caspase-3 activation by the Ginkgo biloba extract EGb761. Proc Natl Acad Sci U S A 2002;99:12197–12202.
Andrieu S, Gillette S, Amouyal K, Nourhashemi F, et al. Association of Alzheimer’s disease onset with ginkgo biloba and other symptomatic cognitive treatments in a population of women aged 75 years and older from the EPIDOS study. J Gerontol A Biol Sci Med Sci 2003;58:372–377.
Schneider LS, DeKosky ST, Farlow MR, et al. A randomized, double-blind, placebocontrolled trial of two doses of Ginkgo biloba extract in dementia of the Alzheimer’s type. Curr Alzheimer Res 2005;2:541–551
Van Dongen M, van Rossum E, Kessels A, et al. Ginkgo for elderly people with dementia and age-associated memory impairment: a randomized clinical trial. J Clin Epidemiol 2003;56:367–376.
Van Dongen MC, van Rossum E, Kessels AG, et al. The efficacy of ginkgo for elderly people with dementia and age-associated memory impairment: new results of a randomized clinical trial. J Am Geriatr Soc 2000;48:1183–1194.
Kanowski S, Hoerr R. Ginkgo biloba extract EGb 761 in dementia: intent-to-treat analyses of a 24-week, multi-center, double-blind, placebo-controlled, randomized trial. Pharmacopsychiatry 2003;36:297–303
Le Bars PL, Kieser M, Itil KZ. A 26-week analysis of a double-blind, placebocontrolled trial of the ginkgo biloba extract EGb 761 in dementia. Dement Geriatr Cogn Disord 2000;11:230–237
Kurz A, Van Baelen B. Ginkgo biloba compared with cholinesterase inhibitors in the treatment of dementia: a review based on meta-analyses by the cochrane collaboration. Dement Geriatr Cogn Disord 2004;18:217–226.
Mazza M, Capuano A, Bria P, et al. Ginkgo biloba and donepezil: a comparison in the treatment of Alzheimer’s dementia in a randomized placebo-controlled doubleblind study. Eur J Neurol 2006;13:981–985
Tauskela JS. MitoQ—a mitochondria-targeted antioxidant. IDrugs 2007;10:399–412.
Wang S, Zhu L, Shi H, et al. Inhibition of melatonin biosynthesis induces neurofilament hyperphosphorylation with activation of cyclin-dependent kinase 5. Neurochem Res 2007;32:1329–1335.
Srinivasan V, Pandi-Perumal SR, Maestroni GJ, Esquifino AI, et al. Role of melatonin in neurodegenerative diseases. Neurotox Res 2005;7:293–318.
Wang JZ, Wang ZF. Role of melatonin in Alzheimer-like neurodegeneration. Acta Pharmacol Sin 2006;27:41–49
Gupta A, Pansari K. Inflammation and Alzheimer’s disease. Int J Clin Pract 2003;57:36–39.
in t’ Veld BA, Ruitenberg A, Hofman A, et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N Engl J Med 2001;345:1515–1521.
Zandi PP, Anthony JC, Hayden KM, et al. Reduced incidence of AD with NSAID but not H2 receptor antagonists: the Cache County Study. Neurology 2002;59:880–886.
Szekely CA, Breitner JC, Fitzpatrick AL, et al. NSAID use and dementia risk in the Cardiovascular Health Study: role of APOE and NSAID type. Neurology 2008; 70:17–24.
Rogers J, Kirby LC, Hempelman SR, et al. Clinical trial of indomethacin in Alzheimer’s disease. Neurology 1993;43:1609–1611.
Tabet N, Feldman H. Indomethacin for Alzheimer’s disease. Cochrane Database of Systematic Reviews 2002;2:CD003673.
de Jong D, Jansen R, Hoefnagels W, et al. No effect of one-year treatment with indomethacin on Alzheimer’s disease progression: a randomized controlled trial. PLoS ONE. 2008;3:e1475.
Tabet N, Feldmand H. Ibuprofen for Alzheimer’s disease. Cochrane Database Syst Rev. 2003;2:CD004031.
Reines SA, Block GA, Morris JC, et al. Rofecoxib: no effect on Alzheimer’s disease in a 1-year, randomized, blinded, controlled study. Neurology 2004;62:66–71.
Soininen H, West C, Robbins J, et al. Long-term efficacy and safety of celecoxib in Alzheimer’s disease. Dement Geriatr Cogn Disord 2007;23:8–21.
Aisen PS, Schafer KA, Grundman M, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA 2003;289:2819–2826.
Geerts H. Drug evaluation: (R)-flurbiprofen—an enantiomer of flurbiprofen for the treatment of Alzheimer’s disease. IDrugs 2007;10:121–133.
Brode S, Cooke A. Immune-potentiating effects of the chemotherapeutic drug cyclophosphamide. Crit Rev Immunol 2008;28:109–126.
Tobinick EL, Gross H. Rapid cognitive improvement in Alzheimer’s disease following perispinal etanercept administration. J Neuroinflammation 2008; Jan 9;5:2.
Harikumar KB, Aggarwal BB. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle 2008;7:1020–1035.
Yang F, Lim GP, Begum AN, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem 2005;280:5892–5901.
Greenamyre JT, Young AB. Excitatory amino acids and Alzheimer’s disease. Neurobiol Aging 1989;10:593–602.
Chohan MO, Iqbal K. From tau to toxicity: emerging roles of NMDA receptor in Alzheimer’s disease. J Alzheimers Dis 2006;10:81–87.
Parsons CG, Stöffler A, Danysz W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system—too little activation is bad, too much is even worse. Neuropharmacology 2007;53:699–723.
Farlow MR. NMDA receptor antagonists. A new therapeutic approach for Alzheimer’s disease. Geriatrics 2004;59:22–27.
Lipton SA. Pathologically-activated therapeutics for neuroprotection: mechanism of NMDA receptor block by memantine and S-nitrosylation. Curr Drug Targets 2007;8:621–632
Zoladz PR, Campbell AM, Park CR, et al. Enhancement of long-term spatial memory in adult rats by the noncompetitive NMDA receptor antagonists, memantine and neramexane. Pharmacol Biochem Behav 2006;85:298–306.
Dicou E, Rangon CM, Guimiot F, et al. Positive allosteric modulators of AMPA receptors are neuroprotective against lesions induced by an NMDA agonist in neonatal mouse brain. Brain Res 2003;970:221–225.
Quirk JC, Nisenbaum ES. LY404187: a novel positive allosteric modulator of AMPA receptors. CNS Drug Rev 2002;8:255–282.
Chappell AS, Gonzales C, Williams J, et al. AMPA potentiator treatment of cognitive deficits in Alzheimer disease. Neurology 2007;68:1008–1012
http://www.clinicaltrials.gov. (accessed 15 december 2010).
Fumagalli F, Racagni G, Riva MA. The expanding role of BDNF: a therapeutic target for Alzheimer’s disease? Pharmacogenomics J 2006;6:8–15
Conner JM, Darracq MA, Roberts J, et al. Nontropic actions of neurotrophins: subcortical nerve growth factor gene delivery reverses age-related degeneration of primate cortical cholinergic innervation. Proc Natl Acad Sci U S A 2001;98:1941–1946.
Capsoni S, Giannotta S, Cattaneo A. Nerve growth factor and galantamine ameliorate early signs of neurodegeneration in anti-nerve growth factor mice. Proc Natl Acad Sci U S A 2002;99:12432–12437.
Tuszynski MH, Thal L, Pay M, et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med 2005;11:551–555.
Grundman M, Capparelli E, Kim HT, et al. A multicenter, randomized, placebo controlled, multiple-dose, safety and pharmacokinetic study of AIT-082 (Neotrofin) in mild Alzheimer’s disease patients. Life Sci 2003;73:539–553.
Sugaya K, Alvarez A, Marutle A, et al. Stem cell strategies for Alzheimer’s disease therapy. Panminerva Med 2006;48:87–96.
Oliveira AA Jr, Hodges HM. Alzheimer’s disease and neural transplantation as prospective cell therapy. Curr Alzheimer Res 2005;2:79–95
Elder GA, De Gasperi R, Gama Sosa MA. Research update: neurogenesis in adult brain and neuropsychiatric disorders. Mt Sinai J Med 2006;73:931–940.
Lovell MA, Geiger H, Van Zant GE, et al. Isolation of neural precursor cells from Alzheimer’s disease and aged control postmortem brain. Neurobiol Aging 2006;27:909–917.
Pagocic V, Herrling P. List of drugs in development for neurodegenerative diseases. Neurodegenerative Dis 2007;4: 443–486
Rees TM, Brimijoin S. The role of acetylcholinesterase in the pathogenesis of Alzheimer’s disease. Drugs Today (Barc) 2003;39:75–83
Ballard CG, Greig NH, Guillozet-Bongaarts AL, et al. Cholinesterases: roles in the brain during health and disease. Curr Alzheimer Res 2005;2:307–318.
Mori E, Hashimoto M, Krishnan KR, et al. What constitutes clinical evidence for neuroprotection in Alzheimer disease: support for the cholinesterase inhibitors? Alzheimer Dis Assoc Disord 2006;20:S19–S26.
Zhang HY, Tang XC. Neuroprotective effects of huperzine A: new therapeutic targets for neurodegenerative disease. Trends Pharmacol Sci 2006;27:619–625.
Li J, Wu HM, Zhou RL, et al. Huperzine A for Alzheimer’s disease. Cochrane Database Syst Rev 2008;2:CD005592.
Wang R, Tang XC. Neuroprotective effects of huperzine A. A natural cholinesterase inhibitor for the treatment of Alzheimer’s disease. Neurosignals 2005;14:71–82.
Greig NH, Sambamurti K, Yu QS, et al. An overview of phenserine tartrate, a novel acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease. Curr Alzheimer Res 2005;2:281–290.
Kadir A, Andreasen N, Almkvist O, et al. Effect of phenserine treatment on brain functional activity and amyloid in Alzheimer’s disease. Ann Neurol 2008;63:621–631.
Lahiri DK, Chen D, Maloney B, et al. The experimental Alzheimer’s disease drug posiphen [(+)-phenserine] lowers amyloid-beta peptide levels in cell culture and mice. J Pharmacol Exp Ther 2007;320:386–396.
Klein J. Phenserine. Expert Opin Investig Drugs 2007;16:1087–1097
Lane RM, Potkin SG, Enz A. Targeting acetylcholinesterase and butyrylcholinesterase in dementia. Int J Neuropsychopharmacol 2006;9:101–124.
Kamal MA, Al-Jafari AA, Yu QS, et al. Kinetic analysis of the inhibition of human butyrylcholinesterase with cymserine. Biochim Biophys Acta 2006;1760:200–206.
Kamal MA, Klein P, Yu QS, et al. Kinetics of human serum butyrylcholinesterase and its inhibition by a novel experimental Alzheimer therapeutic, bisnorcymserine. J Alzheimers Dis 2006;10:43–51
Thal LJ, Forrest M, Loft H, et al. Lu 25–109, a muscarinic agonist, fails to improve cognition in Alzheimer’s disease. Neurology 2000;54:421–426.
Messer WS Jr. The utility of muscarinic agonists in the treatment of Alzheimer’s disease. J Mol Neurosci 2002;19:187–193
isher A, Pittel Z, Haring R, et al. M1 muscarinic agonists can modulate some of the hallmarks in Alzheimer’s disease: implications in future therapy. J Mol Neurosci 2003;20:349–356.
Caccamo A, Oddo S, Billings LM, et al. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron 2006;49:671–682
Wienrich M, Meier D, Ensinger HA, et al. Pharmacodynamic profile of the M1 agonist talsaclidine in animals and man. Life Sci 2001;68:2593–2600.
Hock C, Maddalena A, Raschig A, et al. Treatment with the selective muscarinic m1 agonist talsaclidine decreases cerebrospinal fluid levels of A beta 42 in patients with Alzheimer’s disease. Amyloid 2003;10:1–6.
Arneric SP, Sullivan JP, Decker MW, et al. Potential treatment of Alzheimer disease using cholinergic channel activators (ChCAs) with cognitive enhancement, anxiolytic-like, and cytoprotective properties. Alzheimer Dis Assoc Disord 1995;9:50–61.
Lippiello PM, Bencherif M, Gray JA, et al. RJR-2403: a nicotinic agonist with CNS selectivity II. In vivo characterization. J Pharmacol Exp Ther 1996;279:1422–1429.
Bencherif M, Bane AJ, Miller CH, et al. TC-2559: a novel orally active ligand selective at neuronal acetylcholine receptors. Eur J Pharmacol 2000;409:45–55.
Lippiello P, Letchworth SR, Gatto GJ, et al. Ispronicline: a novel alpha4beta2 nicotinic acetylcholine receptor-selective agonist with cognition-enhancing and neuroprotective properties. J Mol Neurosci 2006;30:19–20
Lin NH, Gunn DE, Ryther KB, et al. Structure-activity studies on 2-methyl-3-(2(S)-pyrrolidinylmethoxy) pyridine (ABT-089): an orally bioavailable 3-pyridyl ether nicotinic acetylcholine receptor ligand with cognition-enhancing properties. J Med Chem 1997;40:385–390
Rueter LE, Anderson DJ, Briggs CA, et al. ABT-089: pharmacological properties of a neuronal nicotinic acetylcholine receptor agonist for the potential treatment of cognitive disorders. CNS Drug Rev 2004;10:167–182.
Kem WR. The brain alpha7 nicotinic receptor may be an important therapeutic target for the treatment of Alzheimer’s disease: studies with DMXBA (GTS-21). Behav Brain Res 2000;113:169–181
Dunbar GC, Inglis F, Kuchibhatla R, et al. Effect of ispronicline, a neuronal nicotinic acetylcholine receptor partial agonist, in subjects with age associated memory impairment (AAMI). J Psychopharmacol 2007;21:171–178.
Williamson JD, Miller ME, Bryan RN, et al. The Action to Control Cardiovascular Risk in Diabetes Memory in Diabetes Study (ACCORD-MIND): rationale, design, and methods. Am J Cardiol 2007;99:112–122.
Rönnemaa E, Zethelius B, Sundelöf J, et al. Impaired insulin secretion increases the risk of Alzheimer disease. Neurology 2008; 71:1065–1071.
Sabayan B, Foroughinia F, Mowla A, et al. Role of Insulin Metabolism Disturbances in the Development of Alzheimer Disease: Mini Review. Am J Alzheimers Dis Other Demen 2008; 23:192–199
Craft S. Insulin resistance and Alzheimer’s disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res 2007;4:147–152.
Gasparini L, Gouras GK, Wang R, et al. Stimulation of beta-amyloid precursor protein trafficking by insulin reduces intraneuronal beta-amyloid and requires mitogen-activated protein kinase signaling. J Neurosci 2001;21:2561–2570.
Reger MA, Watson GS, Green PS, et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology 2008;70:440–448.
Watson GS, Craft S. The role of insulin resistance in the pathogenesis of Alzheimer’s disease: implications for treatment. CNS Drugs 2003;17:27–45.
Watson GS, Cholerton BA, Reger MA, et al. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry 2005;13:950–958.
Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457–2471.
Reger MA, Henderson ST, Hale C, et al. Effects of beta-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol Aging 2004;25:311–314.
http://www.accerapharma.com (accessed 15 december 2008)
Yaffe K, Sawaya G, Lieberburg I, et al. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA 1998;279:688–695.
Hogervorst E, Williams J, Budge M, et al. The nature of the effect of female gonadal hormone replacement therapy on cognitive function in post-menopausal women: a meta-analysis. Neuroscience 2000;101:485–512.
LeBlanc A. Estrogen and Alzheimer’s disease. Curr Opin Investig Drugs 2002;3:768–773.
Maki P, Hogervorst E. The menopause and HRT. HRT and cognitive decline. Best Pract Res Clin Endocrinol Metab 2003;17:105–122.
Webber KM, Perry G, Smith MA, et al. The contribution of luteinizing hormone to Alzheimer disease pathogenesis. Clin Med Res 2007;5:177–183.
Webber KM, Casadesus G, Atwood CS, et al. Gonadotropins: a cohesive gender-based etiology of Alzheimer disease. Mol Cell Endocrinol 2007;260–262:271–275.
Short RA, Bowen RL, O’Brien PC, et al. Elevated gonadotropin levels in patients with Alzheimer disease. Mayo Clin Proc 2001;76:906–909.
Bowen RL, Smith MA, Harris PL, et al. Elevated luteinizing hormone expression colocalizes with neurons vulnerable to Alzheimer’s disease pathology. J Neurosci Res 2002;70:514–518.
Casadesus G, Garrett MR, Webber KM, et al. The estrogen myth: potential use of gonadotropin-releasing hormone agonists for the treatment of Alzheimer’s disease. Drugs R D 2006;7:187–193.
Meethal SV, Smith MA, Bowen RL, et al. The gonadotropin connection in Alzheimer’s disease. Endocrine 2005;26:317–326.
Cherrier MM, Matsumoto AM, Amory JK, et al. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology 2005;64:2063–2068.
Lu PH, Masterman DA, Mulnard R, et al. Effects of testosterone on cognition and mood in male patients with mild Alzheimer disease and healthy elderly men. Arch Neurol 2006;63:177–185.
Cherrier MM, Asthana S, Plymate S, et al. Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology 2001;57:80–88.
Cherrier MM, Matsumoto AM, Amory JK, et al. The role of aromatization in testosterone supplementation: effects on cognition in older men. Neurology 2005;64:290–296.
Merriam GR, Schwartz RS, Vitiello MV. Growth hormone-releasing hormone and growth hormone secretagogues in normal aging. Endocrine 2003;22:41–48.
Vitiello MV, Moe KE, Merriam GR, et al. Growth hormone releasing hormone improves the cognition of healthy older adults. Neurobiol Aging 2006;27:318–323.
Sevigny JJ, Ryan JM, van Dyck CH, et al. Growth hormone secretagogue MK-677: no clinical effect on AD progression in a randomized trial. Neurology. 2008 Nov 18;71(21):1702–1708.
Hibberd C, Yau JL, Seckl JR. Glucocorticoids and the ageing hippocampus. J Anat 2000;197: 553–562.
Green KN, Billings LM, Roozendaal B, et al. Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer’s disease. J Neurosci 2006;26:9047–9056.
Dhikav V, Anand KS. Glucocorticoids may initiate Alzheimer’s disease: a potential therapeutic role for mifepristone (RU-486). Med Hypotheses 2007;68:1088–1092.
Pomara N, Doraiswamy PM, Tun H, et al. Mifepristone (RU 486) for Alzheimer’s disease. Neurology 2002;58:1436.
DeBattista C, Belanoff J. C-1073 (mifepristone) in the adjunctive treatment of Alzheimer’s disease. Curr Alzheimer Res 2005;2:125–129
Komater VA, Buckley MJ, Browman KE, et al. Effects of histamine H3 receptor antagonists in two models of spatial learning. Behav Brain Res 2005;159:295–300.
Medhurst AD, Atkins AR, Beresford IJ, et al. GSK189254, a novel H3 receptor antagonist that binds to histamine H3 receptors in Alzheimer’s disease brain and improves cognitive performance in preclinical models. J Pharmacol Exp Ther 2007;321:1032–1045.
Bachurin S, Bukatina E, Lermontova N, et al. Antihistamine agent Dimebon as a novel neuroprotector and a cognition enhancer. Ann N Y Acad Sci 2001;939:425–435.
Doody RS, Gavrilova SI, Sano M, et al. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer’s disease: a randomised, double-blind, placebo-controlled study. Lancet 2008;372:207–215.
Truchot L, Costes SN, Zimmer L, et al. Up-regulation of hippocampal serotonin metabolism in mild cognitive impairment. Neurology 2007;69:1012–1017
Raje S, Patat AA, Parks V, et al. A positron emission tomography study to assess binding of lecozotan, a novel 5-hydroxytryptamine-1A silent antagonist, to brain 5-HT1A receptors in healthy young and elderly subjects, and in patients with Alzheimer’s disease. Clin Pharmacol Ther 2008;83:86–96.
Childers WE Jr, Abou-Gharbia MA, Kelly MG, et al. Synthesis and biological evaluation of benzodioxanylpiperazine derivatives as potent serotonin 5-HT(1A) antagonists: the discovery of Lecozotan. J Med Chem 2005;48:3467–3470.
Schechter LE, Smith DL, Rosenzweig-Lipson S, et al. Lecozotan (SRA-333): a selective serotonin 1A receptor antagonist that enhances the stimulated release of glutamate and acetylcholine in the hippocampus and possesses cognitive-enhancing properties. J Pharmacol Exp Ther 2005;314:1274–1289.
Labie C, Lafon C, Marmouget C, et al. Effect of the neuroprotective compound SR57746A on nerve growth factor synthesis in cultured astrocytes from neonatal rat cortex. Br J Pharmacol 1999;127:139–144.
Kennedy BP, Ziegler MG, Alford M, et al. Early and persistent alterations in prefrontal cortex MAO A and B in Alzheimer’s disease. J Neural Transm 2003; 110:789–801.
Youdim MB. The path from anti Parkinson drug selegiline and rasagiline to multifunctional neuroprotective anti Alzheimer drugs ladostigil and m30. Curr Alzheimer Res 2006;3:541–550.
Khachaturian AS, Zandi PP, Lyketsos CG, et al. Antihypertensive medication use and incident Alzheimer disease: the Cache County Study. Arch Neurol 2006; 63:686–692.
Kehoe PG, Wilcock GK. Is inhibition of the renin-angiotensin system a new treatment option for Alzheimer’s disease? Lancet Neurol 2007;6:373–378
Hemming ML, Selkoe DJ, Farris W. Effects of prolonged angiotensin-converting enzyme inhibitor treatment on amyloid beta-protein metabolism in mouse models of Alzheimer disease. Neurobiol Dis 2007;26:273–281.
Forette F, Seux ML, Staessen JA, et al. The prevention of dementia with antihypertensive treatment: new evidence from the Systolic Hypertension in Europe (Syst-Eur) study. Arch Intern Med 2002;162:2046–2052
Yasar S, Corrada M, Brookmeyer R, et al. Calcium channel blockers and risk of AD: the Baltimore Longitudinal Study of Aging. Neurobiol Aging 2005;26:157–163.
López-Arrieta JM, Birks J. Nimodipine for primary degenerative, mixed and vascular dementia. Cochrane Database Syst Rev 2002;3:CD000147.
Morris MS. Homocysteine and Alzheimer’s disease. Lancet Neurol 2003;2:425–428.
Seshadri S. Elevated plasma homocysteine levels: risk factor or risk marker for the development of dementia and Alzheimer’s disease? J Alzheimers Dis 2006;9:393–398.
Aisen PS, Schneider LS, Sano M, et al. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA 2008;300:1774–1783.
Chan A, Shea TB. Folate deprivation increases presenilin expression, gammasecretase activity, and Abeta levels in murine brain: potentiation by ApoE deficiency and alleviation by dietary S-adenosyl methionine. J Neurochem 2007;102:753–760.
Pogacic V, Herrling P. list of drugs in development for neurodegenerative diseases. Neurodegenerative Dis 2007,4:443–486
Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M. Alzheimer’s disease: clinical trials and drug development. Lancet Neurol. 2010 Jul;9(7):702–716
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Piau, A., Nourhashémi, F., Hein, C. et al. Progress in the development of new drugs in Alzheimer’s disease. J Nutr Health Aging 15, 45–57 (2011). https://doi.org/10.1007/s12603-011-0012-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-011-0012-x