Abstract
Background
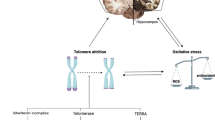
Many studies have shown that short telomere length (TL) is associated with high oxidative stress and various age-related diseases. Parkinson’s disease (PD) is an age-related disease, and although its pathogenic mechanism is uncertain, oxidative stress is believed to be implicated in this pathology. The aim of this case-control study was to assess both TL and the different markers of oxidative stress in elderly patients with PD compared to age control subjects.
Methods
20 PD patients and 15 age-matched controls, >65 years were studied. TL was measured by Southern blotting from DNA samples extracted from white blood cells. Superoxide dismutase (SOD) activity and plasma levels of total glutathione and protein carbonyls were determined.
Results
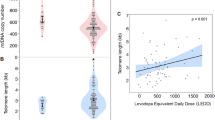
There was a trend for lower TL in PD patients: 6.06 ± 0.81 kb in PD versus 6.45± 0.73 kb in controls (p = 0.08). No significant difference was found between the two groups in terms of oxidative stress markers. In controls, age was the main determinant of telomere shortening (r = −0.547; p = 0.03) whereas, in PD patients, telomere shortening was mainly dependent on plasmatic concentrations of carbonyl proteins (r= −0.544; p=0.044). In PD patients, a negative association was observed between plasma carbonyl protein levels and SOD activity (r= − 0.622, p=0.004).
Conclusions
In PD, TL is shorter in presence of high oxidative stress as measured by carbonyl protein levels. The absence of telomere attrition with age among patients with PD could reflect a telomere regulation by mechanisms other than age.
Similar content being viewed by others
Abbreviations
- BMI:
-
Body Mass Index
- CRP:
-
C-Reactive Protein
- CV:
-
CardioVascular
- DNPH:
-
2, 4-dinitrophenylhydrazine
- DTNB:
-
5,5′-dithiobis-2-nitrobenzoic
- GSH:
-
Glutathione
- HDL-C:
-
High-Density Lipoprotein Cholesterol
- kb:
-
kilobase
- LDL-C:
-
Low-Density Lipoprotein Cholesterol
- n.a:
-
not applicable
- PD:
-
Parkinson’s Disease
- SOD:
-
Superoxide dismutase
- TG:
-
TriGlycerides
- TL:
-
Telomere Length
- TNB:
-
5-thio-2-nitrobenzoic
- TRF:
-
Terminal Restriction Fragment
- WBCs:
-
White Blood Cells
References
Harley CB: Telomere loss: mitotic clock or genetic time bomb? Mutat Res 256: 271–282, 1991
Aviv A: Telomeres and human aging: facts and fibs. Sci Aging Knowledge Environ 2004: pe43, 2004
Cattan V, Mercier N, Gardner JP, et al.: Chronic oxidative stress induces a tissuespecific reduction in telomere length in CAST/Ei mice. Free Radic Biol Med 44: 1592–1598, 2008
Matthews C, Gorenne I, Scott S, et al.: Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ Res 99: 156–164, 2006
von Zglinicki T: Oxidative stress shortens telomeres. Trends Biochem Sci 27: 339–344, 2002
Chang E and Harley CB: Telomere length and replicative aging in human vascular tissues. Proc Natl Acad Sci U S A 92: 11190–11194, 1995
Benetos A, Gardner JP, Zureik M, et al.: Short telomeres are associated with increased carotid atherosclerosis in hypertensive subjects. Hypertension 43: 182–185, 2004
Benetos A, Okuda K, Lajemi M, et al.: Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension 37: 381–385, 2001
Jeanclos E, Schork NJ, Kyvik KO, Kimura M, Skurnick JH and Aviv A: Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension 36: 195–200, 2000
Panossian LA, Porter VR, Valenzuela HF, et al.: Telomere shortening in T cells correlates with Alzheimer’s disease status. Neurobiol Aging 24: 77–84, 2003
Samani NJ, Boultby R, Butler R, Thompson JR and Goodall AH: Telomere shortening in atherosclerosis. Lancet 358: 472–473, 2001
Dexter DT, Holley AE, Flitter WD, et al.: Increased levels of lipid hydroperoxides in the parkinsonian substantia nigra: an HPLC and ESR study. Mov Disord 9: 92–97, 1994
Jenner P and Olanow CW: Oxidative stress and the pathogenesis of Parkinson’s disease. Neurology 47: S161–170, 1996
Wang H, Chen H, Gao X, et al.: Telomere length and risk of Parkinson’s disease. Mov Disord 23: 302–305, 2008
Guan JZ, Maeda T, Sugano M, et al.: A percentage analysis of the telomere length in Parkinson’s disease patients. J Gerontol A Biol Sci Med Sci 63: 467–473, 2008
Dragonas C, Bertsch T, Sieber CC and Brosche T: Plasmalogens as a marker of elevated systemic oxidative stress in Parkinson’s disease. Clin Chem Lab Med 47: 894–897, 2009
Ershler WB: The value of the SENIEUR protocol: distinction between “ideal aging” and clinical reality. Mech Ageing Dev 122: 134–136, 2001
Miller SA, Dykes DD and Polesky HF: A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16: 1215, 1988
Tentolouris N, Nzietchueng R, Cattan V, et al.: White blood cells telomere length is shorter in males with type 2 diabetes and microalbuminuria. Diabetes Care 30: 2909–2915, 2007
Martin-Ruiz C, Dickinson HO, Keys B, Rowan E, Kenny RA and Von Zglinicki T: Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann Neurol 60: 174–180, 2006
Lev N, Melamed E and Offen D: Apoptosis and Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry 27: 245–250, 2003
Nawrot TS, Staessen JA, Gardner JP and Aviv A: Telomere length and possible link to X chromosome. Lancet 363: 507–510, 2004
Barnham KJ, Masters CL and Bush AI: Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov 3: 205–214, 2004
Prigione A, Begni B, Galbussera A, et al.: Oxidative stress in peripheral blood mononuclear cells from patients with Parkinson’s disease: negative correlation with levodopa dosage. Neurobiol Dis 23: 36–43, 2006
Prigione A, Isaias IU, Galbussera A, et al.: Increased oxidative stress in lymphocytes from untreated Parkinson’s disease patients. Parkinsonism Relat Disord 15: 327–328, 2009
Serra JA, Dominguez RO, de Lustig ES, et al.: Parkinson’s disease is associated with oxidative stress: comparison of peripheral antioxidant profiles in living Parkinson’s, Alzheimer’s and vascular dementia patients. J Neural Transm 108: 1135–1148, 2001
Younes-Mhenni S, Frih-Ayed M, Kerkeni A, Bost M and Chazot G: Peripheral blood markers of oxidative stress in Parkinson’s disease. Eur Neurol 58: 78–83, 2007
Martin HL and Teismann P: Glutathione—a review on its role and significance in Parkinson’s disease. FASEB J 23: 3263–3272, 2009
Alam ZI, Daniel SE, Lees AJ, Marsden DC, Jenner P and Halliwell B: A generalised increase in protein carbonyls in the brain in Parkinson’s but not incidental Lewy body disease. J Neurochem 69: 1326–1329, 1997
Sudha K, Rao AV, Rao S and Rao A: Free radical toxicity and antioxidants in Parkinson’s disease. Neurol India 51: 60–62, 2003
Kocaturk PA, Akbostanci MC, Tan F and Kavas GO: Superoxide dismutase activity and zinc and copper concentrations in Parkinson’s disease. Pathophysiology 7: 63–67, 2000
Abraham S, Soundararajan CC, Vivekanandhan S and Behari M: Erythrocyte antioxidant enzymes in Parkinson’s disease. Indian J Med Res 121: 111–115, 2005
Bostantjopoulou S, Kyriazis G, Katsarou Z, Kiosseoglou G, Kazis A and Mentenopoulos G: Superoxide dismutase activity in early and advanced Parkinson’s disease. Funct Neurol 12: 63–68, 1997
Ihara Y, Chuda M, Kuroda S and Hayabara T: Hydroxyl radical and superoxide dismutase in blood of patients with Parkinson’s disease: relationship to clinical data. J Neurol Sci 170: 90–95, 1999
Kurz DJ, Decary S, Hong Y, Trivier E, Akhmedov A and Erusalimsky JD: Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci 117: 2417–2426, 2004
De Meyer T, Rietzschel ER, De Buyzere ML, Van Criekinge W and Bekaert S: Studying telomeres in a longitudinal population based study. Front Biosci 13: 2960–2970, 2008
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Watfa, G., Dragonas, C., Brosche, T. et al. Study of telomere length and different markers of oxidative stress in patients with Parkinson’s disease. J Nutr Health Aging 15, 277–281 (2011). https://doi.org/10.1007/s12603-010-0275-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-010-0275-7