Abstract
The disparity between increased lifespan and healthy aging, marked by prevalent “inflammaging”, highlights the global challenge in care of older persons. This study explored the anti-inflammatory effects of Lactiplantibacillus plantarum HEAL9 (LpHEAL9), alone or combined with berries, on older volunteers with chronic low-grade inflammation (LGI). It was a randomized, double-blind, placebo-controlled trial, with a total of 66 volunteers (> 70 years old), randomly assigned, and equally distributed, to placebo, LpHEAL9 or LpHEAL9 + Berries group. After a 2-week run-in period, participants underwent a 4-week dietary intervention. Intake of LpHEAL9 showed a trend towards reduction in serum CRP but without reaching statistical significance. However, LpHEAL9 significantly decreased fecal calprotectin levels compared to placebo. LpHEAL9+Berries did not show any effect on inflammation. Both probiotic groups showed a trend in improving cognitive function albeit not reaching statistical significance. Our findings suggest that the probiotic strain L. plantarum HEAL9 has a modest impact on LGI in a healthy older population (ClinicalTrials.gov ID: NCT02342496).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
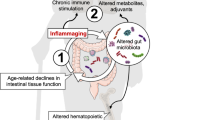
According to projections from the United Nations [1], the population aged 65 years or older is expected to rise from 0.7 billion in 2019 to 1.5 billion by 2050. Despite advances in healthcare practices, this increase in lifespan has not been paralleled by healthy longevity, presenting significant challenges for healthcare systems globally and adversely affecting the quality of life for individuals. Aging is marked by the accumulation of molecular and cellular alterations that contribute to an elevated vulnerability to chronic diseases prevalent in later life [2]. A key factor underlying various age-related conditions, including atherosclerosis, diabetes, cognitive decline, and Alzheimer’s disease, is the presence of systemic low-grade inflammation (LGI), a status referred to as “inflammaging” [3]. The genesis of LGI is complex and not entirely understood, involving but not limited to genetic predispositions, the biological aging of chromosomal telomeres, oxidative stress, lifestyle factors (e.g., high-fat diet, smoking, sedentary behavior), and alterations in gut microbiota [3,4,5,6]. Furthermore, inflammaging is linked to immunosenescence, characterized by the aged immune system’s diminished capacity to generate anti-inflammatory mediators, thereby failing to adequately counteract the release of pro-inflammatory agents, including endotoxins [7, 8].
Emerging evidence indicates an important role of the gut microbiota in contributing to LGI. Altered microbiota has been frequently observed in the metabolic disorders such as obesity and nonalcoholic fatty liver disease (NAFLD), and has been explored as therapeutic target [9]. Aging also brings about significant changes in microbiota composition. For instance, older adults tend to have a decreased proportion of Bacillota (also known as Firmicutes) and an increased proportion of Bacteroidota (also known as Bacteroidetes) and Pseudomonadota (also known as Proteobacteria), along with decreased microbial diversity, compared to younger individuals [10,11,12,13,14]. Such an unfavorable shift could progress to dysbiosis, which in turn leads to increased intestinal permeability to endotoxins (lipopolysaccharides on the surface of Gram-negative bacteria) as well as other bacterial components that can leak into the systemic circulation and eventually precipitate LGI. A worsening of the gut health may also lead to a reduction in nutrient absorption and ultimately resulting in undernourishment of the older individual [15, 16]. Notably, age-associated muscle loss has been linked to increased gut permeability. A recent study reported that a 16-week probiotic intervention repaired intestinal barrier function and improved quality of life measures in older adults suffering muscle decline [17]. Thus, manipulation of the microbiota composition in older population is a promising potential strategy to prevent or reduce age-associated disease and disability, especially when related to low-grade inflammation. Several attempts have been made using probiotics to counteract LGI and have shown promising results. One study found that consuming Lactobacillus delbrueckii subsp. bulgaricus 8481 over a 6-month period enhanced immune function in older adults (age > 65 years). This was achieved by decelerating the aging process in T cell subpopulations and boosting the immature T cells [18]. Another study showed that intake of Lactobacillus casei Shirota strain for 1-month improved innate immune response as measured by natural killer (NK) cell activity in healthy older adults (age 55–74 years) [19]. A dietary intervention incorporating the probiotic VSL#3® containing multiple probiotic strains led to an increase in bifidobacteria, enhanced plasma levels of folate and vitamin B12, and reduced plasma homocysteine levels (marker for oxidative stress) among individuals with low-grade inflammation [20]. Another study testing the effect of multi-strain probiotics coupled with omega-3 showed that 8-week intake of the product increased the anti-inflammatory cytokine IL-10 in older adults with LGI [21]. Moreover, combining probiotics with prebiotics or other functional food ingredients to achieve a synergistic effect presents a highly promising approach to counteract LGI. Ingredients such as antioxidant-rich dark-colored berries have been shown to improve cognitive function in aged rats [22] and counteract oxidative stress in older individuals [23]. One of the bioactive compounds in these berries are tannins which could be degraded by bacterial tannase into smaller phenolic compounds that have anti-inflammatory properties [24].
Considering the strain-specific nature of probiotic functions and their potential in reducing LGI, this study primarily aimed to investigate the anti-inflammatory activity of the probiotic strain Lactiplantibacillus plantarum HEAL9 in older individuals exhibiting moderately elevated serum C-reactive protein (CRP) levels, indicative of LGI. While these individuals were otherwise healthy, the primary aim was to understand the direct impact of the probiotic strain L. plantarum HEAL9 on inflammation markers. L. plantarum HEAL9 was chosen because it has been previously demonstrated to influence the immune response in combination with another strain (Lacticaseibacillus paracasei 8700:2) [25, 26]. Given the significant tannase activity of L. plantarum HEAL9, as a secondary aim, we further examined whether combining this probiotic strain with a mixture of blackberries and blackcurrants enhanced its anti-inflammatory effects, leveraging the bioactive properties of the berries. We employed this dual approach to investigate the probiotic’s standalone benefits and its potential synergistic interactions with polyphenol-rich berries.
Materials and Methods
Design of the Study
This was a randomized, double-blind and placebo-controlled study with the objective to evaluate the benefit of Lactiplantibacillus plantarum HEAL9 (DSM 15312, LpHEAL9, HEAL9™) alone or in combination with lyophilized blackcurrants and blackberries, compared to a placebo, in older individuals with low grade systemic inflammation. The study was conducted in Sweden (ClinicalTrials.gov ID: NCT02342496) and ethical approval was received by the Ethics committee in Lund/Malmö, Sweden (2014/86). All participants provided signed informed consent before randomization into one of the three study groups. This clinical study was performed in compliance with the Declaration of Helsinki as well as the ICH-GCP guidelines and EU recommendations (CPMP/ICH/135/95).
Study Participants
The study involved healthy participants of both genders, aged 70 years and above who had systemic low-grade inflammation (LGI). LGI was defined as a CRP level of 2–10 mg/L. Upon enrollment, participants should be able to fill in the study diary daily and understand any oral/written study-related information provided to them. Exclusion criteria included the presence of chronic inflammatory diseases such as IBD and rheumatism, ongoing corticosteroid treatment, and intake of antibiotic treatment in the last 4 weeks prior to inclusion in the study.
Study Procedures
The study participants were recruited through a database with subjects that had previously taken part in the Epidemiology for Health (EpiHealth) study and had approved of being contacted again for participation in other studies. Following a screening visit to confirm CRP levels, eligible subjects were randomly allocated into one of the three study groups. They entered a 2-week wash-out period during which they refrained from using other probiotic products, and they started to fill in the study diary. After the wash-out period a baseline visit was scheduled for distributing the study product and ensuring the study diary was correctly completed. The participants were instructed not to consume other probiotic products all through the study period. They then returned to the clinic for the final visit 28 days later. The study product was consumed once daily, ideally at breakfast, and the study diary was filled in every day, during the whole intervention period. The parameters recorded in the diary were fecal consistency (Bristol stool form scale), experience of flatulence (none, medium, high) and abdominal pain (none, medium, high). Blood pressure and body weight were measured at each study visit. Plasma and serum samples were collected at baseline and at end of study for analyses of CRP, fibrinogen, ALAT, ASAT, GT, ALP, e-GFR, creatinine, and total protein by Labmedicin Skåne (University and regional laboratories in Region Skåne, Sweden). Additional blood samples were stored at – 80 °C for future analysis of additional blood markers. Serum levels of IFN-γ, IL-10, IL-1β, IL-6, TNF-α, MCP-1, and fractalkine were analyzed using U-PLEX Human assays from MSD (U-PLEX Human, Meso Scale Diagnostics, Rockville, MD, USA) according to the manufacturer’s instructions. Fecal samples were also collected at baseline and at end of study for the analyses of calprotectin (Department of Clinical Chemistry at Skåne University Hospital, Malmö, Sweden) and zonulin using a competitive enzyme-linked immunosorbent assay (ELISA) kit (Immundiagnostik AG, Bensheim, Germany), following the manufacturer’s protocol.
At the start and end of the study, all participants were asked to fill in a quality-of-life questionnaire (SF-36) [27]. The SF-36 is divided into eight multi-items, 0–100 scored scales (physical functioning, role limitations-physical, bodily pain, general health, vitality, social functioning, role limitations-emotional, and mental health). The higher the score, the more positive the health status. Participants’ cognitive function was assessed using the validated Trail Making Test (TMT) [28]. TMT-A determines cognitive processing speed and the subjects had to connect circles labeled with numbers 1–25 in an ascending order. TMT-B measures executive functions and the participants alternated between numbers (1–13) and letters (A-L) in an ascending order (i.e., 1-A-2-B and so forth). A computerized version of TMT was used and administered at baseline and after 28 days intervention (end of study). A computer mouse was used to join the symbols and an erroneous path was indicated by changing its color to red. The total time required to perform the test correctly was measured in seconds. Any adverse events were reported by the participants at the study visits and documented in the case report forms (CRF).
Investigational Product
The investigational product (IP) was produced in the form of a powder with 10 g per sachet as the daily dose. The active IP consisted of Lactiplantibacillus plantarum HEAL9 (LpHEAL9), at 1 × 1010 CFU/sachet with or without the addition of a mixture of freeze-dried blackberries and black currants (6 g freeze-dried powder, consisting of 3 g of each berry, derived from 43 g of fresh berries, MOLDA AG, Dahlenburg, Germany). Red beet extract and flavor (Nordarom AB, Norrköping, Sweden) were added to give the study products without berries the same appearance, taste, and texture as the IP with the berries and maltodextrin was used as a filler. The content from each sachet was consumed once daily after mixing with sour milk or pouring over the breakfast flakes. The study participants were randomly allocated to receive one of the three study products (placebo, LpHEAL9, or LpHEAL9 + Berries) using a computer-generated randomization list with blocks of three and the ratio of 1:1:1. Sealed envelopes were prepared for the allocation concealment and were safely stored by the investigators throughout the study. The labelling of the study product and the preparation of the sealed code envelopes were done by personnel not otherwise involved in any study-related activities. Both study participants and study personnel were blinded to the identity of the study product. Compliance was verified based on the daily records of consumption given in the study diary.
Outcomes
The primary aim of this study was to investigate the impact of L. plantarum HEAL9 with or without the berries in comparison to placebo, on the changes in CRP levels from baseline to end of study. The secondary objectives included evaluating differences in blood and fecal markers between and within the groups, as measured from baseline to end of study, as well as any changes in blood pressure, body weight, fecal consistency, experience of flatulence, abdominal pain, quality of life, cognition, and adverse events reported.
Statistical Analyses
The statistical analysis was performed by an independent statistician using StatXact Version 11.1.0, and STATA Version 16. Since no earlier study existed to base the sample size calculation on, it was decided to include about 20 participants per group. The non-parametric Wilcoxon rank sum test was applied for the between groups analysis of continuous variables and when needed Student’s t test was used to check the robustness. Fischer’s exact test was used for the categorical endpoints. Analysis of within-group changes was done using Wilcoxon signed rank test for continuous variables and McNemar test for categorical variables. All participants having consumed at least one dose of the investigational products, i.e., the intention to treat (ITT) population, were included in the analysis of safety parameters. The analysis of efficacy parameters was based on the per protocol group (PP) that in this case included all participants from the ITT group except for one participant that had consumed antibiotics during the study. For the serum inflammatory marker analysis, three other participants were excluded due to medical reasons. All presented p values are nominal, i.e., not adjusted for multiplicity, and p values less than 5% are considered statistically significant.
Results
A total of 394 subjects were contacted to participate in the current study and 187 were screened for eligibility (Fig. 1). In total, 66 participants were included and randomized, 22 participants in each of the three study groups: placebo, LpHEAL9, or LpHEAL9 + Berries. All participants except for two in the LpHEAL9 group completed the study as planned. The reasons for the early terminations were abdominal discomfort in one case and a drop-out for unknown reasons in the other.
There were no differences at baseline between the probiotic groups and the placebo group in terms of gender, age, BMI, and smoking habits or intake of medication (Table 1). The mean age of the subjects was 73.2 years, with a range from 70 to 79 years. Most participants were men (65%) and the mean BMI value of the subjects was 27.8 kg/m2 (range 20–43 kg/m2). The compliance was good; four participants did not take the study product for 1 day and one participant in the placebo group missed taking the study product for 3 days. Four participants commented that the volume of the study products was large while the rest of the participants did not have any problem with the amount.
Anti-Inflammatory Activity
CRP levels decreased in the LpHEAL9 group compared to the placebo group but did not reach statistical significance (Table 2, p = 0.22). There was a trend of decreasing CRP levels within the LpHEAL9 group (Baseline vs. End of study; median [IQR]: 2.85 [1.68, 5.08] vs. 2.30 [1.13, 3.15] mg/L, p = 0.1).
Fecal calprotectin levels decreased significantly after the consumption of LpHEAL9 compared to placebo (Table 2, Fig. 2). Moreover, there was a significant difference (p = 0.01) in the number of participants with increased calprotectin values at end of study compared to baseline in the placebo group (11/22) versus the LpHEAL9 group (2/17). No significant differences were found within the groups in calprotectin levels for baseline versus end of study.
Fecal calprotectin levels in the elderly participants. Changes in the fecal calprotectin levels from baseline to the end of study showed a significant reduction in LpHEAL9 group compared to placebo (A). Measured calprotectin levels at baseline and end of study in each group (B). No significant difference was detected within-group comparisons
No significant differences were identified between the probiotic groups and the placebo group regarding any changes in fibrinogen and total protein measured in serum, or zonulin measured in feces (Table 2). The possible anti-inflammatory activity of the probiotic bacteria was further assessed in serum with the analyses of IFN-g, IL-10, IL-1b, IL-6, TNF-a, MCP-1, and fractalkine. No significant differences were identified in the probiotic groups compared to the placebo group for any of these markers (Table 3). However, in terms of change from baseline to end of study in the levels of TNF-a, the reduction in the LpHEAL9 group (median [IQR], − 0.10 [− 0.37, 0.52]) pg/ml tended (p = 0.07) to differ from that seen in the placebo group (median [IQR], 0.09 [− 0.21, 0.60] pg/ml).
Gastrointestinal Symptoms, Quality of Life, and Safety Markers
There were no differences between the probiotic groups and the placebo group in number of bowel movements at start or at any other measured timepoints in the study (data not shown). For the LpHEAL9 group the number of bowel movements increased significantly at week 2 of the intervention compared to baseline but this difference was not sustained in the following weeks (Fig. S1A). In the same group, a significant decrease in the mean weekly number of normal stools (i.e., the sum of Bristol stool types 3, 4, and 5) compared to baseline was observed at week 1, 2, and 3. No significant changes in stool consistency compared to baseline were observed in the other study groups (Fig. S1B).
The SF-36 questionnaire was used to evaluate quality of life, where a higher value indicates a better health status (Table S1). The social functioning decreased in all three groups, with a significant decrease within the LpHEAL9 (p < 0.05) and a trend of reduction in the LpHEAL9 + Berries group (p < 0.1). However, there were no statistically significant differences found between the groups. Bodily pain was significantly improved (p < 0.05) within the LpHEAL9 + Berries group, and the improvement from baseline to end of study was significantly larger compared to the placebo group.
There were no alterations in the level of flatulence, abdominal pain, liver (ASAT, ALAT, GT, ALP) or kidney function (eGFR) and body weight, in any of the groups during the study period (Table S2). In terms of blood pressure, no change over time was detected in the two probiotic groups, whereas there was a significant reduction seen for SBP in the placebo group. This resulted in a significant difference in the change of SBP between LpHEAL9 group and placebo group (p = 0.01) (Table S2).
Cognitive Function
The cognitive function was evaluated at baseline and end of the study (week 4) with trail making test A (TMT-A) and B (TMT-B) (Table S3). The time for making the TMT-A correctly at baseline among all participants ranged from 28 to 208 s and for TMT-B the range was from 35 to 237 s with mean values of 47.49 and 74.71 s, respectively. No significant differences were detected in the between-group comparisons. However, a trend toward improved cognitive function over time was observed within the LpHEAL9 group, as evidenced by a decrease in the time required to perform TMT-A (p = 0.092). A similar trend was observed for TMT-B in the LpHEAL9 + Berries group (p = 0.067).
Discussion
This study aimed to assess the anti-inflammatory effects of the probiotic strain L. plantarum HEAL9 (LpHEAL9), either standalone or in combination with lyophilized berries, in older individuals with low-grade systemic inflammation (LGI). Chronic LGI is a recognized characteristic of inflammaging, yet standard biomarkers to definitively indicate the presence of this LGI state are lacking [29]. Consequently, researchers often rely on a variety of biomarkers, with standard CRP or high-sensitivity CRP (hs-CRP) as a common endpoint to identify LGI. Our findings indicate a trend towards reduced CRP levels in the LpHEAL9 group compared to the placebo group, hinting at a potential anti-inflammatory role for this probiotic strain. Nevertheless, the absence of statistical significance calls for larger future studies to determine the efficacy of LpHEAL9 in lowering CRP levels. To note, at baseline—2 weeks post-inclusion and prior to the intake of study products—approximately 36% of participants (32% in both the LpHEAL9 and LpHEAL9 + Berries groups, and 45% in the placebo group) exhibited CRP levels below 2 mg/L, indicating a lack of low-grade inflammation at the intervention’s outset, which poses a limitation to our study. Previous studies have shown a more pronounced reduction in hs-CRP levels following probiotic supplementation when participants were stratified by an hs-CRP ≥ 3 mg/L cutoff, indicative of LGI [20]. This demonstrates a critical influence of baseline inflammatory status on research outcomes and could partially explain why we did not see an effect on CRP. On the other hand, a modest sample size of the present study restricted our ability to perform detailed subgroup analyses, underlining the inherent difficulties of relying on CRP levels for defining low-grade systemic inflammation. Additionally, the within person variability of CRP levels in a short timeframe, also observed with hs-CRP [18, 23], further complicates the assessment. This variability highlights the necessity for more precisely defined biomarkers to accurately define the low-grade inflammation state.
Intake of LpHEAL9 for 4 weeks resulted in significantly reduced levels of fecal calprotectin in comparison with the placebo group. Further analysis showed that significantly more participants in the placebo group, compared to the LpHEAL9 group, had an increase in the calprotectin level. In the context of chronic inflammation, elevated fecal calprotectin level can reflect both neutrophil migration through an inflamed gut wall and upregulated expression of the calprotectin by intestinal epithelial cells in response to inflammatory stimuli [30]. Notably, fecal calprotectin was superior in distinguishing between non-inflammatory and inflammatory bowel disease compared to CRP [31,32,33]. A 1-month intervention study reported that 15 days of consuming the Mediterranean diet did not affect fecal calprotectin levels in older obese women, but combining the Mediterranean diet with multi-strain probiotics during the following 15 days led to a significant reduction in fecal calprotectin levels [34]. Another study involving middle aged individuals with functional diarrhea and elevated fecal calprotectin demonstrated that the administration of a different L. plantarum strain (CJLP243) over an 8-week period significantly reduced calprotectin levels from baseline [35]. Concurrently, a greater proportion of participants in the probiotic group experienced substantial relief from functional diarrhea (measured as more than 30% reduction in the frequency of loose stools) compared to those in the placebo group [35]. On the other hand, administration of probiotic strain Bacillus coagulans GBI-30, 6086 had no effect on fecal calprotectin level in older participants but increased anti-inflammatory cytokine IL-10 [36]. These inconsistencies in reducing calprotectin level via probiotics intervention could be due to the strain dependent effect or, as already mentioned, to variation in baseline inflammatory status in the studied population. While universal reference values for inflammation are not established, fecal calprotectin level below 40 mg/kg suggests a ≤ 1% chance of inflammatory bowel disease [27]. For the calprotectin measurement technique utilized in this study, a reference value of < 50 mg/kg was adopted, with 63% of participants exhibiting values beneath this threshold at baseline.
We hypothesized that by combining tannase-producing LpHEAL9 with tannin-rich blackberries and blackcurrants would result in smaller phenolic compounds with anti-oxidative and anti-inflammatory capacity [24, 37], which in turn could deliver extra benefit. However, in participants consuming the LpHEAL9 + Berries there was no obvious benefit in CRP levels, calprotectin or other inflammatory markers compared to the participants that consumed LpHEAL9 alone. The amount of freeze-dried berries in the daily dose of the IP corresponded to 43 g fresh berries and this may be a too low dose to observe an effect on inflammation. A study on obese subjects with increased LDL cholesterol levels, showed that consuming high dose strawberry powders (32 g/day, equivalent to ~ 375 g of fresh strawberries) but not low dose (equivalent to one serving daily of ~ 150 g fresh strawberries), reduced the level of the an inflammatory marker serum plasminogen activator inhibitor-1 (PAI-1) [38].
Proinflammatory cytokine TNF-α is another frequently measured marker in intervention studies targeting inflammation. Although there is no consistent evidence of probiotic effect on TNF-α [18, 20, 21, 36, 39, 40], a meta-analysis on probiotics effect on inflammatory markers found that probiotic consumption could significantly reduce TNF-α, and also CRP and IL-6 [41]. In the current study, a trend was observed toward a reduction in the proinflammatory TNF-α in LpHEAL9 group compared to placebo.
In the current study, we have observed tendencies towards improved performance in trail making tests used to examine visual attention and executive functioning within both probiotic groups. A recent finding also showed that the same probiotic strain as used in the current study, LpHEAL9, enhanced cognitive functions, mood, and sleep in moderately stressed but otherwise healthy individuals aged 21 to 52 years [42]. The small sample size and low statistical power might contribute to lack of significant differences between groups. There is a growing interest in exploring the gut-brain axis as a means to understand how altering the gut microbiota can impact neurological function [43, 44]. This interest is driven by the potential of using gut microbiota modulation as a non-pharmacological strategy to combat cognitive decline that often accompanies aging. It has been reported that 12-weeks intake of probiotics containing bifidobacteria strains led to an improvement in mental flexibility and mood in healthy older persons [45].
Regarding product safety, our findings contribute to the growing body of evidence supporting the safety of probiotic supplementation in older populations. The overall good tolerance reported by most participants, alongside the absence of significant changes in gastrointestinal symptoms, liver and kidney function, and on body weight across all study groups, demonstrate the safety profile of LpHEAL9, both alone and in combination with lyophilized berries. This is particularly relevant given the heightened vulnerability of the older persons to adverse health effects from supplements and the critical importance of ensuring safety in interventions targeting this population.
Conclusion
In summary, a 4-week intake of LpHEAL9 significantly decreased levels of fecal calprotectin in this group of otherwise healthy older subjects, indicating an anti-inflammatory effect. Additionally, the LpHEAL9 probiotic strain has shown promising trends toward reducing CRP and TNF-α levels and improving cognitive function, underscoring its potential to support healthy aging by modulating inflammation. The inclusion of tannin-rich blackberries and blackcurrants with the LpHEAL9 strain did not provide the expected synergistic benefits. This could be due to the relatively low dose of berries used in the study, potentially limiting their ability to enhance the probiotic’s effects. Further research is necessary to fully explore the impact of the LpHEAL9 strain on low-grade inflammation and its benefits for healthy aging, including investigations into optimal dosages of berry supplementation for achieving synergistic effects.
Data Availability
Supporting data for the findings of this study are available upon request from the corresponding author.
References
Nations U (2020) World population ageing 2019 (st/esa/ser. a/444). Department of Economic and Social Affairs PD, editor New York, USA2020
Li X, Li C, Zhang W, Wang Y, Qian P, Huang H (2023) Inflammation and aging: signaling pathways and intervention therapies. Signal Transduct Target Ther 8(1):239. https://doi.org/10.1038/s41392-023-01502-8
Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G (2000) Inflamm-aging: an evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908(1):244–254
Chakravarti D, LaBella KA, DePinho RA (2021) Telomeres: history, health, and hallmarks of aging. Cell 184(2):306–322. https://doi.org/10.1016/j.cell.2020.12.028
Dugan B, Conway J, Duggal NA (2023) Inflammaging as a target for healthy ageing. Age Ageing 52(2). https://doi.org/10.1093/ageing/afac328
Hajam YA, Rani R, Ganie SY, Sheikh TA, Javaid D, Qadri SS, Pramodh S, Alsulimani A, Alkhanani MF, Harakeh S, Hussain A, Haque S, Reshi MS (2022) Oxidative stress in human pathology and aging: molecular mechanisms and perspectives. Cells 11(3). https://doi.org/10.3390/cells11030552
Kim KA, Jeong JJ, Yoo SY, Kim DH (2016) Gut microbiota lipopolysaccharide accelerates inflamm-aging in mice. BMC Microbiol 16:9. https://doi.org/10.1186/s12866-016-0625-7
Jin CJ, Baumann A, Brandt A, Engstler AJ, Nier A, Hege M, Schmeer C, Kehm R, Höhn A, Grune T, Witte OW, Bergheim I (2020) Aging-related liver degeneration is associated with increased bacterial endotoxin and lipopolysaccharide binding protein levels. Am J Physiol-Gastr L 318(4):G736–G747. https://doi.org/10.1152/ajpgi.00345.2018
Tarantino G, Finelli C (2015) Systematic review on intervention with prebiotics/probiotics in patients with obesity-related nonalcoholic fatty liver disease. Future Microbiol 10(5):889–902. https://doi.org/10.2217/fmb.15.13
Mariat D, Firmesse O, Levenez F, Guimarăes V, Sokol H, Doré J, Corthier G, Furet J (2009) The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol 9(1):1–6
Mäkivuokko H, Tiihonen K, Tynkkynen S, Paulin L, Rautonen N (2010) The effect of age and non-steroidal anti-inflammatory drugs on human intestinal microbiota composition. Brit J Nutr 103(2):227–234. https://doi.org/10.1017/S0007114509991553
Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, Marchesi JR, Falush D, Dinan T, Fitzgerald G, Stanton C, van Sinderen D, O’Connor M, Harnedy N, O’Connor K, Henry C, O’Mahony D, Fitzgerald AP, Shanahan F, Twomey C, Hill C, Ross RP, O’Toole PW (2011) Composition, variability, and temporal stability of the intestinal microbiota of the elderly. P Natl Acad Sci USA 108:4586–4591. https://doi.org/10.1073/pnas.1000097107
Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkila J, Monti D, Satokari R, Franceschi C, Brigidi P, De Vos W (2010) Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One 5(5):e10667. https://doi.org/10.1371/journal.pone.0010667
Wang XM, Fan L, Meng CC, Wang YJ, Deng LE, Yuan Z, Zhang JP, Li YY, Lv SC (2024) Gut microbiota influence frailty syndrome in older adults: mechanisms and therapeutic strategies. Biogerontology 25(1):107–129. https://doi.org/10.1007/s10522-023-10082-7
Krajmalnik-Brown R, Ilhan ZE, Kang DW, DiBaise JK (2012) Effects of gut microbes on nutrient absorption and energy regulation. Nutr Clin Pract 27(2):201–214. https://doi.org/10.1177/0884533611436116
Muñoz-Fernandez SS, Garcez FB, Alencar JC, Bastos AA, Morley JE, Cederholm T, Aprahamian I, de Souza HP, Avelino-Silva TJ, Bindels LB (2024) Gut microbiota disturbances in hospitalized older adults with malnutrition and clinical outcomes. Nutrition 112369. https://doi.org/10.1016/j.nut.2024.112369
Qaisar R, Burki A, Karim A, Iqbal MS, Ahmad F (2024) Probiotics supplements Improve the sarcopenia-related quality of life in older adults with age-related muscle decline. Calcif Tissue Int 114(6):583–591. https://doi.org/10.1007/s00223-024-01211-6
Moro-Garcia MA, Alonso-Arias R, Baltadjieva M, Fernandez Benitez C, Fernandez Barrial MA, Diaz Ruisanchez E, Alonso Santos R, Alvarez Sanchez M, Saavedra Mijan J, Lopez-Larrea C (2013) Oral supplementation with Lactobacillus delbrueckii subsp. bulgaricus 8481 enhances systemic immunity in elderly subjects. Age (Dordr) 35(4):1311–1326. https://doi.org/10.1007/s11357-012-9434-6
Dong H, Rowland I, Thomas LV, Yaqoob P (2013) Immunomodulatory effects of a probiotic drink containing Lactobacillus casei Shirota in healthy older volunteers. Eur J Nutr 52(8):1853–1863. https://doi.org/10.1007/s00394-012-0487-1
Valentini L, Pinto A, Bourdel-Marchasson I, Ostan R, Brigidi P, Turroni S, Hrelia S, Hrelia P, Bereswill S, Fischer A, Leoncini E, Malaguti M, Blanc-Bisson C, Durrieu J, Spazzafumo L, Buccolini F, Pryen F, Donini LM, Franceschi C, Lochs H (2015) Impact of personalized diet and probiotic supplementation on inflammation, nutritional parameters and intestinal microbiota - The “RISTOMED project”: randomized controlled trial in healthy older people. Clin Nutr 34(4):593–602. https://doi.org/10.1016/j.clnu.2014.09.023
Tingo L, Hutchinson AN, Bergh C, Stiefvatter L, Schweinlin A, Jensen MG, Kruger K, Bischoff SC, Brummer RJ (2022) Potential modulation of inflammation by probiotic and omega-3 supplementation in elderly with chronic low-grade inflammation—a randomized, placebo-controlled trial. Nutrients 14(19). https://doi.org/10.3390/nu14193998
Shukitt-Hale B, Cheng V, Joseph JA (2009) Effects of blackberries on motor and cognitive function in aged rats. Nutr Neurosci 12(3):135–140. https://doi.org/10.1179/147683009X423292
McGhie TK, Walton MC, Barnett LE, Vather R, Martin H, Au J, Alspach PA, Booth CL, Kruger MC (2007) Boysenberry and blackcurrant drinks increased the plasma antioxidant capacity in an elderly population but had little effect on other markers of oxidative stress. J Sci Food Agric 87(13):2519–2527
Landete J (2011) Ellagitannins, ellagic acid and their derived metabolites: a review about source, metabolism, functions and health. Food Res Int 44(5):1150–1160
Berggren A, Lazou Ahrén I, Larsson N, Önning G (2011) Randomised, double-blind and placebo-controlled study using new probiotic lactobacilli for strengthening the body immune defence against viral infections. Eur J Nutr 50:203–210
Busch R, Gruenwald J, Dudek S (2013) Randomized, Double Blind and Placebo Controlled Study Using a Combination of Two Probiotic Lactobacilli to Alleviate Symptoms and Frequency of Common Cold. FNS 04:13–20. https://doi.org/10.4236/fns.2013.411A003
Sullivan M, Karlsson J, Ware JE Jr (1995) The Swedish SF-36 Health Survey—I. Evaluation of data quality, scaling assumptions, reliability and construct validity across general populations in Sweden. Soc Sci Med 41(10):1349–1358
Bowie CR, Harvey PD (2006) Administration and interpretation of the Trail Making Test. Nat Protoc 1(5):2277–2281
Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N, Slavich GM (2019) Chronic inflammation in the etiology of disease across the life span. Nat Med 25(12):1822–1832. https://doi.org/10.1038/s41591-019-0675-0
Jukic A, Bakiri L, Wagner EF, Tilg H, Adolph TE (2021) Calprotectin: from biomarker to biological function. Gut 70(10):1978–1988. https://doi.org/10.1136/gutjnl-2021-324855
Schoepfer AM, Trummler M, Seeholzer P, Seibold-Schmid B, Seibold F (2008) Discriminating IBD from IBS: comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies. Inflamm Bowel Dis 14(1):32–39. https://doi.org/10.1002/ibd.20275
Menees SB, Powell C, Kurlander J, Goel A, Chey WD (2015) A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am J Gastroenterol 110(3):444–454. https://doi.org/10.1038/ajg.2015.6
Desai D, Faubion WA, Sandborn WJ (2007) Review article: biological activity markers in inflammatory bowel disease. Aliment Pharmacol Ther 25(3):247–255. https://doi.org/10.1111/j.1365-2036.2006.03184.x
Cancello R, Turroni S, Rampelli S, Cattaldo S, Candela M, Cattani L, Mai S, Vietti R, Scacchi M, Brigidi P, Invitti C (2019) Effect of short-term dietary intervention and probiotic mix supplementation on the gut microbiota of elderly obese women. Nutrients 11(12). https://doi.org/10.3390/nu11123011
Jung M, Jung S, Kim N, Ahn H, Yun H, Kim KN (2022) A randomized, double-blind, placebo-controlled trial to assess the efficacy and safety of Lactiplantibacillus plantarum CJLP243 in patients with functional diarrhea and high fecal calprotectin levels. Nutrients 14(2). https://doi.org/10.3390/nu14020389
Nyangale EP, Farmer S, Cash HA, Keller D, Chernoff D, Gibson GR (2015) Bacillus coagulans GBI-30, 6086 Modulates Faecalibacterium prausnitzii in Older Men and Women. J Nutr 145(7):1446–1452. https://doi.org/10.3945/jn.114.199802
Rippin Sharma AK, Beniwal V (2023) Reconnoitring the antioxidant and antibacterial potential of different fruits after tannin acyl hydrolase mediated biotransformation. Biotechnol Appl Biochem 70(4):1439–1449. https://doi.org/10.1002/bab.2461
Basu A, Izuora K, Betts NM, Kinney JW, Salazar AM, Ebersole JL, Scofield RH (2021) Dietary strawberries improve cardiometabolic risks in adults with obesity and elevated serum LDL cholesterol in a randomized controlled crossover trial. Nutrients 13(5). https://doi.org/10.3390/nu13051421
Neto JV, de Melo CM, Ribeiro SM (2013) Effects of three-month intake of synbiotic on inflammation and body composition in the elderly: a pilot study. Nutrients 5(4):1276–1286. https://doi.org/10.3390/nu5041276
Macfarlane S, Cleary S, Bahrami B, Reynolds N, Macfarlane GT (2013) Synbiotic consumption changes the metabolism and composition of the gut microbiota in older people and modifies inflammatory processes: a randomised, double-blind, placebo-controlled crossover study. Aliment Pharmacol Ther 38(7):804–816. https://doi.org/10.1111/apt.12453
Faghfouri AH, Afrakoti L, Kavyani Z, Nogourani ZS, Musazadeh V, Jafarlou M, Dehghan P (2023) The role of probiotic supplementation in inflammatory biomarkers in adults: an umbrella meta-analysis of randomized controlled trials. Inflammopharmacology 31(5):2253–2268. https://doi.org/10.1007/s10787-023-01332-8
Onning G, Montelius C, Hillman M, Larsson N (2023) Intake of Lactiplantibacillus plantarum HEAL9 improves cognition in moderately stressed subjects: a randomized controlled study. Nutrients 15(15). https://doi.org/10.3390/nu15153466
Bialecka-Debek A, Granda D, Szmidt MK, Zielinska D (2021) Gut microbiota, probiotic interventions, and cognitive function in the elderly: a review of current knowledge. Nutrients 13(8). https://doi.org/10.3390/nu13082514
Tahmasbi F, Mirghafourvand M, Shamekh A, Mahmoodpoor A, Sanaie S (2022) Effects of probiotic supplementation on cognitive function in elderly: a systematic review and Meta-analysis. Aging Ment Health 26(9):1778–1786. https://doi.org/10.1080/13607863.2021.1966743
Kim CS, Cha L, Sim M, Jung S, Chun WY, Baik HW, Shin DM (2021) Probiotic Supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: a randomized, double-blind, placebo-controlled, multicenter trial. J Gerontol A Biol Sci Med Sci 76(1):32–40. https://doi.org/10.1093/gerona/glaa090
Acknowledgements
The authors thank Ingrid Palmquist for excellent assistance.
Funding
Open access funding provided by Lund University. Probi AB, Medicinska Fakulteten, Lunds Universitet, Formas, 2011-306.
Author information
Authors and Affiliations
Contributions
Conceptualization, I.L.A., B.J.; methodology, I.L.A., M.B., G.M., S.E., B.J.; formal analysis, I.L.A., S.E., G.Ö., J.X.; writing—original draft preparation, I.L.A.; writing—review and editing, I.L.A., M.B., G.M., G.Ö., J.X., S.E., B.J. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
I.L.A., M.B. and G.Ö. were/are employees of Probi AB. The other authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lazou-Ahrén, I., Björklund, M., Molin, G. et al. Probiotic-Reduced Inflammaging in Older Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. Probiotics & Antimicro. Prot. (2024). https://doi.org/10.1007/s12602-024-10310-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s12602-024-10310-7