Abstract
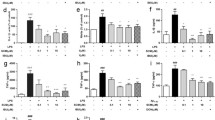
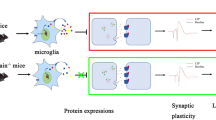
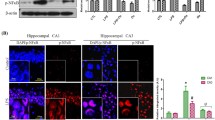
Neuroinflammation is considered an important factor that leads to cognitive impairment. Microglia play a crucial role in neuroinflammation, which leads to cognitive impairment. This study aimed at determining whether temporin-GHaR peptide (GHaR) could improve cognitive function and at uncovering the underlying mechanisms. We found that GHaR treatment alleviated LPS-induced cognitive impairment and inhibited activation of microglia in LPS-induced mice. Furthermore, GHaR inhibited activation of endoplasmic reticulum stress (ERS) and the NF-κB signaling pathway in LPS-induced mice. In vitro, GHaR inhibited M1 polarization of BV2 cells and suppressed TNF-α and IL-6 secretion. Additionally, GHaR neuronal cell viability and apoptosis were induced by LPS-activated microglia-conditioned medium. Moreover, in LPS-induced BV2 cells, GHaR inhibited activation of ERS and the NF-κB signaling pathway. In summary, GHaR improved LPS-induced cognitive and attenuated inflammatory responses via microglial activation reversal. In conclusion, the neuroprotective effects of GHaR were mediated via the ERS signaling pathway.








Similar content being viewed by others
Data Availability
Data will be made available on request.
References
Leng F, Edison P (2021) Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol 17(3):157–172. https://doi.org/10.1038/s41582-020-00435-y
Wang T, Shi C, Luo H, Zheng H, Fan L, Tang M, Su Y, Yang J, Mao C, Xu Y (2022) Neuroinflammation in Parkinson’s Disease: Triggers, Mechanisms, and Immunotherapies. Neuroscientist 28(4):364–381. https://doi.org/10.1177/1073858421991066
Krukowski K, Nolan A, Becker M, Picard K, Vernoux N, Frias ES, Feng X, Tremblay ME, Rosi S (2021) Novel microglia-mediated mechanisms underlying synaptic loss and cognitive impairment after traumatic brain injury. Brain Behav Immun 98:122–135. https://doi.org/10.1016/j.bbi.2021.08.210
Witcher KG, Bray CE, Chunchai T, Zhao F, O’Neil SM, Gordillo AJ, Campbell WA, McKim DB, Liu X, Dziabis JE, Quan N, Eiferman DS, Fischer AJ, Kokiko-Cochran ON, Askwith C, Godbout JP (2021) Traumatic Brain Injury Causes Chronic Cortical Inflammation and Neuronal Dysfunction Mediated by Microglia. J Neurosci 41(7):1597–1616. https://doi.org/10.1523/JNEUROSCI.2469-20.2020
Woodburn SC, Bollinger JL, Wohleb ES (2021) The semantics of microglia activation: neuroinflammation, homeostasis, and stress. J Neuroinflammation 18(1):258. https://doi.org/10.1186/s12974-021-02309-6
Inta D, Lang UE, Borgwardt S, Meyer-Lindenberg A, Gass P (2017) Microglia Activation and Schizophrenia: Lessons From the Effects of Minocycline on Postnatal Neurogenesis, Neuronal Survival and Synaptic Pruning. Schizophr Bull 43(3):493–496. https://doi.org/10.1093/schbul/sbw088
Kwon HS, Koh SH (2020) Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener 9(1):42. https://doi.org/10.1186/s40035-020-00221-2
He Y, Gao Y, Zhang Q, Zhou G, Cao F, Yao S (2020) IL-4 Switches Microglia/macrophage M1/M2 Polarization and Alleviates Neurological Damage by Modulating the JAK1/STAT6 Pathway Following ICH. Neuroscience 437:161–171. https://doi.org/10.1016/j.neuroscience.2020.03.008
Li L, Gan H, Jin H, Fang Y, Yang Y, Zhang J, Hu X, Chu L (2021) Astragaloside IV promotes microglia/macrophages M2 polarization and enhances neurogenesis and angiogenesis through PPARγ pathway after cerebral ischemia/reperfusion injury in rats. Int Immunopharmacol 92:107335. https://doi.org/10.1016/j.intimp.2020.107335
Kardani K, Bolhassani A (2021) Antimicrobial/anticancer peptides: bioactive molecules and therapeutic agents. Immunotherapy 13(8):669–684. https://doi.org/10.2217/imt-2020-0312
Zeng ZZ, Huang SH, Alezra V, Wan Y (2021) Antimicrobial peptides: triumphs and challenges. Future Med Chem 13(16):1313–1315. https://doi.org/10.4155/fmc-2021-0134
He X, Yang S, Wei L, Liu R, Lai R, Rong M (2013) Antimicrobial peptide diversity in the skin of the torrent frog. Amolops jingdongensis Amino Acids 44(2):481–487. https://doi.org/10.1007/s00726-012-1358-z
Kaneider NC, Djanani A, Wiedermann CJ (2007) Heparan sulfate proteoglycan-involving immunomodulation by cathelicidin antimicrobial peptides LL-37 and PR-39. ScientificWorldJournal 7:1832–1838. https://doi.org/10.1100/tsw.2007.285
Mohammed ESI, Radey R (2021) Immunomodulation of Antimicrobial Peptides Expression in the Gastrointestinal Tract by Probiotics in Response to Stimulation by Salmonella minnesota Lipopolysaccharides. Probiotics Antimicrob Proteins 13(4):1157–1172. https://doi.org/10.1007/s12602-021-09746-y
Dong Z, Luo W, Zhong H, Wang M, Song Y, Deng S, Zhang Y (2017) Molecular cloning and characterization of antimicrobial peptides from skin of Hylarana guentheri. Acta Biochim Biophys Sin (Shanghai) 49(5):450–457. https://doi.org/10.1093/abbs/gmx023
Wei H, Xie Z, Tan X, Guo R, Song Y, Xie X, Wang R, Li L, Wang M, Zhang Y (2020) Temporin-Like Peptides Show Antimicrobial and Anti-Biofilm Activities against Streptococcus mutans with Reduced Hemolysis. Molecules. https://doi.org/10.3390/molecules25235724
Zhang F, Zhang JG, Yang W, Xu P, Xiao YL, Zhang HT (2018) 6-Gingerol attenuates LPS-induced neuroinflammation and cognitive impairment partially via suppressing astrocyte overactivation. Biomed Pharmacother 107:1523–1529. https://doi.org/10.1016/j.biopha.2018.08.136
Kim J, Lee HJ, Park JH, Cha BY, Hoe HS (2022) Nilotinib modulates LPS-induced cognitive impairment and neuroinflammatory responses by regulating P38/STAT3 signaling. J Neuroinflammation 19(1):187. https://doi.org/10.1186/s12974-022-02549-0
Heinbockel L, Weindl G, Martinez-de-Tejada G, Correa W, Sanchez-Gomez S, Bárcena-Varela S, Goldmann T, Garidel P, Gutsmann T, Brandenburg K (2018) Inhibition of Lipopolysaccharide- and Lipoprotein-Induced Inflammation by Antitoxin Peptide Pep19–2.5. Front Immunol 9:1704. https://doi.org/10.3389/fimmu.2018.01704
Sousa NA, Oliveira GAL, de Oliveira AP, Lopes ALF, Iles B, Nogueira KM, Araújo TSL, Souza LKM, Araújo AR, Ramos-Jesus J, Plácido A, Amaral C, Campelo YDM, Barbosa EA, Portugal CC, Socodato R, Lobo A, Relvas J, Bemquerer M, Eaton P, Leite J, Medeiros JVR (2020) Novel Ocellatin Peptides Mitigate LPS-induced ROS Formation and NF-kB Activation in Microglia and Hippocampal Neurons. Sci Rep 10(1):2696. https://doi.org/10.1038/s41598-020-59665-1
Shang D, Liang H, Wei S, Yan X, Yang Q, Sun Y (2014) Effects of antimicrobial peptide L-K6, a temporin-1CEb analog on oral pathogen growth, Streptococcus mutans biofilm formation, and anti-inflammatory activity. Appl Microbiol Biotechnol 98(20):8685–8695. https://doi.org/10.1007/s00253-014-5927-9
Qin C, Fan WH, Liu Q, Shang K, Murugan M, Wu LJ, Wang W, Tian DS (2017) Fingolimod Protects Against Ischemic White Matter Damage by Modulating Microglia Toward M2 Polarization via STAT3 Pathway. Stroke 48(12):3336–3346. https://doi.org/10.1161/STROKEAHA.117.018505
Luo G, Wang X, Cui Y, Cao Y, Zhao Z, Zhang J (2021) Metabolic reprogramming mediates hippocampal microglial M1 polarization in response to surgical trauma causing perioperative neurocognitive disorders. J Neuroinflammation 18(1):267. https://doi.org/10.1186/s12974-021-02318-5
Hetz C, Saxena S (2017) ER stress and the unfolded protein response in neurodegeneration. Nat Rev Neurol 13(8):477–491. https://doi.org/10.1038/nrneurol.2017.99
Haile Y, Deng X, Ortiz-Sandoval C, Tahbaz N, Janowicz A, Lu JQ, Kerr BJ, Gutowski NJ, Holley JE, Eggleton P, Giuliani F, Simmen T (2017) Rab32 connects ER stress to mitochondrial defects in multiple sclerosis. J Neuroinflammation 14(1):19. https://doi.org/10.1186/s12974-016-0788-z
Markovinovic A, Greig J, Martín-Guerrero SM, Salam S, Paillusson S (2022) Endoplasmic reticulum-mitochondria signaling in neurons and neurodegenerative diseases. J Cell Sci. https://doi.org/10.1242/jcs.248534
Di Conza G, Ho PC (2020) ER Stress Responses: An Emerging Modulator for Innate Immunity. Cells. https://doi.org/10.3390/cells9030695
Wu Chuang A, Kepp O, Kroemer G, Bezu L (2020) Endoplasmic reticulum stress in the cellular release of damage-associated molecular patterns. Int Rev Cell Mol Biol 350:1–28. https://doi.org/10.1016/bs.ircmb.2019.11.006
Wang YW, Zhou Q, Zhang X, Qian QQ, Xu JW, Ni PF, Qian YN (2020) Correction to: Mild endoplasmic reticulum stress ameliorates lipopolysaccharide-induced neuroinflammation and cognitive impairment via regulation of microglial polarization. J Neuroinflammation 17(1):353. https://doi.org/10.1186/s12974-020-01990-3
Ismael S, Wajidunnisa SK, McDonald MP, Liao FF, Ishrat T (2021) ER stress associated TXNIP-NLRP3 inflammasome activation in hippocampus of human Alzheimer’s disease. Neurochem Int 148:105104. https://doi.org/10.1016/j.neuint.2021.105104
Hosoi T, Ozawa K (2012) Molecular approaches to the treatment, prophylaxis, and diagnosis of Alzheimer’s disease: endoplasmic reticulum stress and immunological stress in pathogenesis of Alzheimer’s disease. J Pharmacol Sci 118(3):319–324. https://doi.org/10.1254/jphs.11r09fm
Wang J, Ding Y, Zhuang L, Wang Z, Xiao W, Zhu J (2021) Ginkgolide B-induced AMPK pathway activation protects astrocytes by regulating endoplasmic reticulum stress, oxidative stress and energy metabolism induced by Aβ1-42. Mol Med Rep. https://doi.org/10.3892/mmr.2021.12096
Funding
This study was supported by grants from the National Natural Science Foundation of China (82104129 to R.W. and 82260250 to DQ.Z.), the Hainan provincial natural science foundation of China (322RC584 to R.W., 820MS140 to DQ.Z.), the Hainan Province Science and Technology Special Fund, Key Research and Development Project of Hainan Province (ZDYF2022SHFZ294 to DQ.Z. and ZDYF2021SHFZ042 to YX.Z.), the Project supported by Hainan Province Clinical Medical Center, and the Foundation of Collaborative Innovation Center of One Health of Hainan University (XTCX2022JKB05 to YX.Z.).
Author information
Authors and Affiliations
Contributions
Da-Qi Zhang: Performed the experiments; Analyzed and interpreted the data; Wrote the paper. Xiaoqian Dong, Simin Su, Linlin Zhang,Jiayu Zhang and Wenjing Yang: Performed the experiments; Analyzed and interpreted the data. Wenting Hu, Lushuang Li , Yanting Song, Xi Xie and Qifu Li: Analyzed and interpreted the data ; review & editing the paper. Rong Wang, Yingxia Zhang: Conceived and designed the experiments.All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, DQ., Dong, X., Su, S. et al. Temporin-GHaR Peptide Alleviates LPS-Induced Cognitive Impairment and Microglial Activation by Modulating Endoplasmic Reticulum Stress. Probiotics & Antimicro. Prot. (2024). https://doi.org/10.1007/s12602-024-10277-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s12602-024-10277-5