Abstract
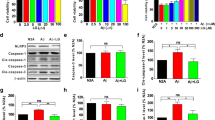
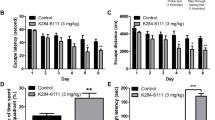
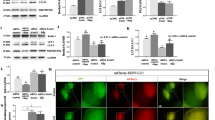
Neuroinflammation is the important pathological feature of Alzheimer’s disease (AD). Legumain, a lysosomal cysteine protease, plays an important role in neuroinflammation during ischemic stroke and depressive disorder. Legumain is involved in AD process through cleaving APP; however, it is unclear if legumain can possibly modulate neuroinflammation without cleaving APP in AD. Thus, we established a mouse model of AD by single intracerebroventricular injections of Aβ1–42 in legumain knockout (KO) mice. The behavioral tests showed that legumain-KO effectively ameliorated cognitive impairment induced by Aβ1–42. Moreover, legumain deprivation significantly improves the synaptic plasticity damages in Aβ1–42-treated mice. Moreover, legumain-KO considerably inhibited the activation of microglia and reduced the expression of inflammatory cytokines in the hippocampus of Aβ1–42-treated mice. Interestingly, we found that legumain-KO inhibited TLR4/MyD88/NF-κB pathway, which was activated by Aβ1–42 in the hippocampus. In conclusion, our results suggested that legumain-KO reduced the level of neuroinflammation that was associated with inhibiting TLR4/MyD88/NF-κB pathways, thereby improving the hippocampal synaptic plasticity and reducing the cognitive impairments in Aβ1–42-treated mice.
Graphical abstract
Legumain knockout blocked microglia activation by inhibiting TLR4/MyD88/NF-κB signaling pathways, and further reduced inflammatory cytokine expression. As a result, legumain knockout alleviated synaptic damage and cognitive impairment induced by Aβ1–-42.







Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Winblad B, Amouyel P, Andrieu S, Ballard C, Brayne C, Brodaty H, Cedazo-Minguez A, Dubois B et al (2016) Defeating Alzheimer’s disease and other dementias: a priority for European science and society. Lancet Neurol 15(5):455–532. https://doi.org/10.1016/s1474-4422(16)00062-4
Mudher A, Lovestone S (2002) Alzheimer’s disease – do tauists and baptists finally shake hands?
Wang H, Jiang T, Li W, Gao N, Zhang T (2018) Resveratrol attenuates oxidative damage through activating mitophagy in an in vitro model of Alzheimer’s disease. Toxicol Lett 282:100–108. https://doi.org/10.1016/j.toxlet.2017.10.021
Fu WY, Wang X, Ip NY (2019) Targeting neuroinflammation as a therapeutic strategy for Alzheimer’s disease: mechanisms, drug candidates, and new opportunities. ACS Chem Neurosci 10(2):872–879. https://doi.org/10.1021/acschemneuro.8b00402
Brosseron F, Traschutz A, Widmann CN, Kummer MP, Tacik P, Santarelli F, Jessen F, Heneka MT (2018) Characterization and clinical use of inflammatory cerebrospinal fluid protein markers in Alzheimer’s disease. Alzheimers Res Ther 10(1):25. https://doi.org/10.1186/s13195-018-0353-3
Strohmeyer R, Rogers J (2001) Molecular and cellular mediators of Alzheimer’s disease inflammation. J Alzheimers Dis 3(1):131–157. https://doi.org/10.3233/JAD-2001-3118
Szekely CA, Town T, Zandi PP (2007) NSAIDs for the chemoprevention of Alzheimer’s disease. Subcell Biochem 42:229–248. https://doi.org/10.1007/1-4020-5688-5_11
Torika N, Asraf K, Apte RN, Fleisher-Berkovich S (2018) Candesartan ameliorates brain inflammation associated with Alzheimer’s disease. CNS Neurosci Ther 24(3):231–242. https://doi.org/10.1111/cns.12802
Liu C, Sun C, Huang H, Janda K, Edgington T (2003) Overexpression of legumain in tumors is significant for invasion/metastasis and a candidate enzymatic target for prodrug therapy. Cancer Res 63(11):2957–2964. https://doi.org/10.1097/00002820-200306000-00012
Zhang Z, Kang SS, Liu X, Ahn EH, Zhang Z, He L, Iuvone PM, Duong DM et al (2017) Asparagine endopeptidase cleaves alpha-synuclein and mediates pathologic activities in Parkinson’s disease. Nat Struct Mol Biol 24(8):632–642. https://doi.org/10.1038/nsmb.3433
Lian J, Li K, Gao J, Tan X, Yang Z (2019) Legumain acts on neuroinflammatory to affect CUS-induced cognitive impairment. Behav Brain Res 376:112219. https://doi.org/10.1016/j.bbr.2019.112219
Ishizaki T, Erickson A, Kuric E, Shamloo M, Hara-Nishimura I, Inacio AR, Wieloch T, Ruscher K (2010) The asparaginyl endopeptidase legumain after experimental stroke. J Cereb Blood Flow Metab 30(10):1756–1766. https://doi.org/10.1038/jcbfm.2010.39
Liu Z, Jang SW, Liu X, Cheng D, Peng J, Yepes M, Li XJ, Matthews S et al (2008) Neuroprotective actions of PIKE-L by inhibition of SET proteolytic degradation by asparagine endopeptidase. Mol Cell 29(6):665–678. https://doi.org/10.1016/j.molcel.2008.02.017
Zhang Z, Xie M, Ye K (2016) Asparagine endopeptidase is an innovative therapeutic target for neurodegenerative diseases. Expert Opin Ther Targets 20(10):1237–1245. https://doi.org/10.1080/14728222.2016.1182990
Zhang Z, Song M, Liu X, Su Kang S, Duong DM, Seyfried NT, Cao X, Cheng L et al (2015) Delta-secretase cleaves amyloid precursor protein and regulates the pathogenesis in Alzheimer’s disease. Nat Commun 6:8762. https://doi.org/10.1038/ncomms9762
Zhang Z, Obianyo O, Dall E, Du Y, Fu H, Liu X, Kang SS, Song M et al (2017) Inhibition of delta-secretase improves cognitive functions in mouse models of Alzheimer’s disease. Nat Commun 8:14740. https://doi.org/10.1038/ncomms14740
Walter S, Letiembre M, Liu Y, Heine H, Penke B, Hao W, Bode B, Manietta N et al (2007) Role of the toll-like receptor 4 in neuroinflammation in Alzheimer’s disease. Cell Physiol Biochem 20(6):947–956. https://doi.org/10.1159/000110455
Ewald SE (2010) Activation of nucleic acid-sensing toll-like receptors requires cleavage by endolysosomal proteases: a mechanism to avoid autoimmunity. Dissertations & Theses Gradworks
Ali T, Kim MO (2015) Melatonin ameliorates amyloid beta-induced memory deficits, tau hyperphosphorylation and neurodegeneration via PI3/Akt/GSk3β pathway in the mouse hippocampus. J Pineal Res 59(1):47–59
Goncalves FQ, Lopes JP, Silva HB, Lemos C, Silva AC, Goncalves N, Tome AR, Ferreira SG et al (2019) Synaptic and memory dysfunction in a beta-amyloid model of early Alzheimer’s disease depends on increased formation of ATP-derived extracellular adenosine. Neurobiol Dis 132:104570. https://doi.org/10.1016/j.nbd.2019.104570
Ahmad A, Ali T, Park HY, Badshah H, Rehman SU, Kim MO (2017) Neuroprotective effect of fisetin against amyloid-beta-induced cognitive/synaptic dysfunction, neuroinflammation, and neurodegeneration in adult mice. Mol Neurobiol 54(3):2269–2285. https://doi.org/10.1007/s12035-016-9795-4
Gao J, Li K, Du L, Yin H, Tan X, Yang Z (2018) Deletion of asparagine endopeptidase reduces anxiety- and depressive-like behaviors and improves abilities of spatial cognition in mice. Brain Res Bull 142:147–155. https://doi.org/10.1016/j.brainresbull.2018.07.010
Ali T, Kim MJ, Rehman SU, Ahmad A, Kim MO (2017) Anthocyanin-loaded PEG-gold nanoparticles enhanced the neuroprotection of anthocyanins in an Abeta1-42 mouse model of Alzheimer’s disease. Mol Neurobiol 54(8):6490–6506. https://doi.org/10.1007/s12035-016-0136-4
Morris RGM (1981) Spatial localization does not require the presence of local cues. Learn Motiv 12(2):0–260. https://doi.org/10.1016/0023-9690(81)90020-5
Malenka RC, Nicoll RA (1999) Long-term potentiation--a decade of progress? Science 285(5435):1870–1874. https://doi.org/10.1126/science.285.5435.1870
Kumari E, Li K, Yang Z, Zhang T (2020) Tacrine accelerates spatial long-term memory via improving impaired neural oscillations and modulating GAD isomers including neuro-receptors in the hippocampus of APP/PS1 AD mice. Brain Res Bull 161:166–176. https://doi.org/10.1016/j.brainresbull.2020.05.007
Wang S, Li K, Zhao S, Zhang X, Yang Z, Zhang J, Zhang T (2020) Early-stage dysfunction of hippocampal theta and gamma oscillations and its modulation of neural network in a transgenic 5xFAD mouse model. Neurobiol Aging 94:121–129. https://doi.org/10.1016/j.neurobiolaging.2020.05.002
Squire LR, Stark CE, Clark RE (2004) The medial temporal lobe. Annu Rev Neurosci 27:279–306. https://doi.org/10.1146/annurev.neuro.27.070203.144130
Teyler TJ, Discenna P (1987) Long-term potentiation. Annu Rev Neurosci 10(1):131–161. https://doi.org/10.1146/annurev.ne.10.030187.001023
Qi Y, Hu NW, Rowan MJ (2013) Switching off LTP: mGlu and NMDA receptor-dependent novelty exploration-induced depotentiation in the rat hippocampus. Cereb Cortex 23(4):932–939. https://doi.org/10.1093/cercor/bhs086
Ingelsson M, Fukumoto H, Newell KL, Growdon JH, Hedley-Whyte ET, Frosch MP, Albert MS, Hyman BT et al (2004) Early Abeta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology 62(6):925–931. https://doi.org/10.1212/01.wnl.0000115115.98960.37
Boza-Serrano A, Ruiz R, Sanchez-Varo R, Garcia-Revilla J, Yang Y, Jimenez-Ferrer I, Paulus A, Wennstrom M et al (2019) Galectin-3, a novel endogenous TREM2 ligand, detrimentally regulates inflammatory response in Alzheimer’s disease. Acta Neuropathol 138(2):251–273. https://doi.org/10.1007/s00401-019-02013-z
Hickman SE, Allison EK, El Khoury J (2008) Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci 28(33):8354–8360. https://doi.org/10.1523/JNEUROSCI.0616-08.2008
Liu S, Liu Y, Hao W, Wolf L, Kiliaan AJ, Penke B, Rube CE, Walter J et al (2012) TLR2 is a primary receptor for Alzheimer’s amyloid beta peptide to trigger neuroinflammatory activation. J Immunol 188(3):1098–1107. https://doi.org/10.4049/jimmunol.1101121
McGeer PL, McGeer EG (2002) Local neuroinflammation and the progression of Alzheimer’s disease. J Neuro-Oncol 8(6):529–538. https://doi.org/10.1080/13550280290100969
Block ML, Hong JS (2005) Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol 76(2):77–98. https://doi.org/10.1016/j.pneurobio.2005.06.004
Liu L, Chan C (2014) IPAF inflammasome is involved in interleukin-1beta production from astrocytes, induced by palmitate; implications for Alzheimer’s disease. Neurobiol Aging 35(2):309–321. https://doi.org/10.1016/j.neurobiolaging.2013.08.016
Kitazawa M, Cheng D, Tsukamoto MR, Koike MA, Wes PD, Vasilevko V, Cribbs DH, LaFerla FM (2011) Blocking IL-1 signaling rescues cognition, attenuates tau pathology, and restores neuronal beta-catenin pathway function in an Alzheimer’s disease model. J Immunol 187(12):6539–6549. https://doi.org/10.4049/jimmunol.1100620
Helfgott DC, Tatter SB, Santhanam U, Clarick RH, Bhardwaj N, May LT, Sehgal PB (1989) Multiple forms of IFN-beta 2/IL-6 in serum and body fluids during acute bacterial infection. J Immunol 142(3):948–953
Blum-Degen D, Muller T, Kuhn W, Gerlach M, Riederer P (1995) Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci Lett 202:17–20. https://doi.org/10.1016/0304-3940(95)12192-7
Lourenco MV, Clarke JR, Frozza RL, Bomfim TR, Forny-Germano L, Batista AF, Sathler LB, Brito-Moreira J et al (2013) TNF-alpha mediates PKR-dependent memory impairment and brain IRS-1 inhibition induced by Alzheimer’s beta-amyloid oligomers in mice and monkeys. Cell Metab 18(6):831–843. https://doi.org/10.1016/j.cmet.2013.11.002
McCoy MK, Tansey MG (2008) TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation 5:45. https://doi.org/10.1186/1742-2094-5-45
Nguyen MD, Julien JP, Rivest S (2002) Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci 3(3):216–227. https://doi.org/10.1038/nrn752
Morris GP, Clark IA, Zinn R, Vissel B (2013) Microglia: a new frontier for synaptic plasticity, learning and memory, and neurodegenerative disease research. Neurobiol Learn Mem 105:40–53. https://doi.org/10.1016/j.nlm.2013.07.002
Malika B, Rivka R, Djordje G, van Noort JM (2002) Broad expression of toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol 11:11–1021. https://doi.org/10.1093/jnen/61.11.1013
Olson JK, Miller SD (2004) Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol 173(6):3916–3924. https://doi.org/10.4049/jimmunol.1t73.6.3916
Minoretti P, Gazzaruso C, Vito CD, Emanuele E, Bianchi M, Coen E, Reino M, Geroldi D (2006) Effect of the functional toll-like receptor 4 Asp299Gly polymorphism on susceptibility to late-onset Alzheimer’s disease. Neurosci Lett 391(3):147–149. https://doi.org/10.1016/j.neulet.2005.08.047
Letiembre M, Hao W, Liu Y, Walter S, Mihaljevic I, Rivest S, Hartmann T, Fassbender K (2007) Innate immune receptor expression in normal brain aging. Neuroscience 146(1):248–254. https://doi.org/10.1016/j.neuroscience.2007.01.004
Wang X, Stridh L, Li W, Dean J, Elmgren A, Gan L, Eriksson K, Hagberg H et al (2009) Lipopolysaccharide sensitizes neonatal hypoxic-ischemic brain injury in a MyD88-dependent manner. J Immunol 183(11):7471–7477. https://doi.org/10.4049/jimmunol.0900762
Rahimifard M, Maqbool F, Moeini-Nodeh S, Niaz K, Abdollahi M, Braidy N, Nabavi SM, Nabavi SF (2017) Targeting the TLR4 signaling pathway by polyphenols: a novel therapeutic strategy for neuroinflammation. Ageing Res Rev 36:11–19. https://doi.org/10.1016/j.arr.2017.02.004
Acknowledgments
We would like to acknowledge this work to our lab team Ph. D fellow Dr. Ekta kumara, who is a native English speaker, for her valuable comments and help.
Funding
This work was supported by grants from the National Natural Science Foundation of China (32070988).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiment: Runwen Chen, Tao Zhang; performed the experiments: Runwen Chen, Qiyue Zhang, Yuxing Yan, Yuying Zhang; analyzed the data: Runwen Chen; wrote the manuscript: Runwen Chen, Yuying Zhang, Tao Zhang; and wrote revised version of manuscript: Runwen Chen, Tao Zhang.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
All individual participants have consented to the submission of the manuscript to the journal.
Ethical Approval
All procedures performed in the study involving animals were in accordance with the ethical standards of the institution or practice. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 121 kb)
Rights and permissions
About this article
Cite this article
Chen, R., Zhang, Q., Yan, Y. et al. Legumain Knockout Protects Against Aβ1–42-Induced AD-like Cognitive Deficits and Synaptic Plasticity Dysfunction Via Inhibiting Neuroinflammation Without Cleaving APP. Mol Neurobiol 58, 1607–1620 (2021). https://doi.org/10.1007/s12035-020-02219-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02219-3