Abstract
Objectives
Oral mucositis (OM) is an acute and highly prevalent side effect of cancer treatments. Currently, there is no effective strategy for its prevention or treatment. This systematic review aimed to assess the effectiveness of biotics used as a therapeutic strategy for the management of OM.
Materials and Methods
The PRISMA checklist was followed and PubMed, Web of Science, and Scopus were screened for clinical and pre-clinical studies assessing the potential effects of biotics in OM. Inclusion criteria included in vivo studies related to oral mucositis evaluating the effect of biotics, and written in Portuguese, English, French, Spanish, or Dutch. The following exclusion criteria were used: systematic reviews and meta-analyses, reviews, case reports, opinion papers or comments, conference papers, letters without results, articles not related to oral therapy-induced mucositis or biotics, or in vitro articles that do not simulate oral mucositis.
Results
From a total of 1250 articles retrieved, 9 were included in this systematic review. Four clinical studies reported a reduction in oral mucositis occurrence with Lactobacillus species (Lactobacillus casei and Lactobacillus brevis CD2) and Bacillus clausii UBBC07. In pre-clinical studies, Lactococcus lactis genetically modified and Lactobacillus reuteri reduced the severity of OM and Streptococcus salivarius K12 also decreased the size of the ulcers.
Conclusion
The findings of this systematic review suggest that probiotic supplementation may potentially reduce the incidence of therapy-induced OM and decrease its severity in patients undergoing cancer treatment. However, the available evidence is marred by significant heterogeneity across studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral mucositis (OM) is an acute and highly prevalent side effect of cancer treatments, consisting of inflamed, erosive, or ulcerative lesions on the oral mucosa [1]. It is the result of a complex and dynamic combination of biological events, involving multiple pathways and interactions between cancer therapy and oral tissues [2]. According to a five-step pathogenesis model suggested by Sonis [3], radiation and chemotherapeutic drugs encourage tissue inflammation and cell apoptosis by producing harmful reactive oxygen species (Step 1 - Initiation) and activating transcription factors such as nuclear factor-B (Step 2 - Primary response). As a result, this will trigger a series of inflammatory pathways and cause proinflammatory cytokines to be upregulated (Step 3 - Amplification), culminating in ulceration (Step 4 - Ulceration). This step resolves when the extracellular matrix sends signals to the epithelium that impact cell proliferation and differentiation (Step 5 - Healing). The submucosa is then re-established, but not exactly to its prior state because of the mucotoxic injury inflicted by cancer therapy.
Although the incidence and severity of oral mucositis widely vary among patients and treatments prescribed, the mean incidence was reported to be approximately 80%, with several patients suffering from severe oral mucositis [4]. Patients who develop oral mucositis experience severe pain which interferes with their nutrition, quality of life (QOL), and ultimately, compliance with their treatment plan [5]. It has also been reported that patients with OM have twice the risk of developing infections and four times the risk of death compared to patients without OM [6]. The degree and duration of oral mucositis are related to the type of chemotherapy or radiation dose used, the volume of tissue treated, and the treatment duration [6].
Changes in the oral microbiome are also known to influence the incidence and severity of OM. This state of altered bacterial colonization associated with disease expression it is known as oral dysbiosis. Dysbiosis can be caused by genetic and environmental factors such as antibiotic use, diet alterations, stress, and chronic diseases [7]. The dominance of opportunistic microorganisms, such as Candida spp. and gram-negative bacteria, increases during cancer therapy and may further aggravate the inflammatory response [8].
According to the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology Clinical Practice Guidelines for Oral Mucositis [9], it is possible to mitigate the risk of developing OM by proceeding with prophylactic oral care, cryotherapy, anti-inflammatory agents (e.g. benzydamine mouthwash), photobiomodulation therapy (e.g. low-level laser therapy), and antimicrobial and coating agents [9]. In terms of the usual clinical interventions to minimize the impact of OM, these include basic oral care, the use of photobiomodulation, anesthetics (e.g. 2% viscous lidocaine mouth rinse), diet modification, and systemic opiates [6].
Despite these guidelines, the management of oral mucositis remains mostly symptomatic and there is no effective strategy for its prevention or treatment [10]. As so, the manipulation of the oral microbiome with biotics – probiotics, prebiotics, postbiotics, and symbiotics - emerged as an alternative treatment or co-adjuvant option. According to the World Health Organization (WHO), probiotics are defined as live microorganisms that confer a health benefit for the host when administered in adequate amounts. Besides probiotics, prebiotics are dietary molecules that promote the growth of beneficial bacteria, postbiotics are microbial metabolites that have beneficial effects, and symbiotics are a combination of pre-, pro-, or postbiotics [11]. Recently, there has been an increasing interest in their use to prevent, mitigate, or treat specific diseases, such as acute infectious diarrhea in infants [12] and periodontal disease [13].
Regardless of the positive effects of biotics in other diseases, the effect of the use of biotics on the management of therapy-induced oral mucositis in cancer patients is yet to be unveiled. Given this scenario, this paper aims to systematically revise the effectiveness of biotics as an alternative therapeutic strategy for the management of oral mucositis.
Materials and Methods
Protocol and Registration
This review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist and registered on the PROSPERO website, CRD42022314339.
Information Sources and Search Strategy
To fulfill the goal of this systematic review, a PICO (population, intervention, comparison, and outcomes) question was formulated: What is the effect of biotics, compared to not using biotics, on the management of therapy-induced oral mucositis in cancer patients?
To develop this review, three databases were used: Pubmed, Scopus, and Web of Science, using the following search query: “(mucositis[MeSH Terms] OR oral mucosit* OR oromucosit*) AND (probiotics[MeSH Terms] OR prebiotics[MeSH Terms] OR probiotic* OR pro-biotic* OR prebiotic* OR pre-biotic* OR postbiotic* OR post-biotic* OR symbiotic* OR Lactobacillus OR Bifidobacterium OR Streptococcus OR Enterococcus OR Saccharomyces OR Lactococcus)”. Searches were conducted on December 14th, 2022.
Eligibility Criteria
Inclusion criteria included studies related to oral mucositis, in vivo studies (in humans and animals), evaluating the effect of pre-, pro-, post-, and symbiotics, and written in Portuguese, English, French, Spanish, or Dutch.
The exclusion criteria were the following: systematic reviews and meta-analyses, reviews, case reports, opinion papers or comments, conference papers, letters without results, articles not related to oral therapy-induced mucositis, unrelated to pre-, pro-, post- or symbiotics, or in vitro articles that do not simulate oral mucositis.
Selection Process
After removing duplicates, the titles and abstracts of the retrieved publications were independently reviewed by two reviewers (LF and IM). Studies not excluded in the screening phase were fully read and full-text analysis was independently conducted by the same reviewers. Any divergence was solved in discussion with a third-party (MJA and BSM). A total of 1250 articles were retrieved from bibliographic databases (PubMed, Scopus, and Web of Science). The study selection process is described in Fig. 2.
Workflow of the study selection process. Reason 1: Systematic reviews and Meta-analyses, Reviews, Case Reports, Opinion papers or comments, Conference papers, and Letters without results; Reason 2: Not written in English, Portuguese, French, Spanish or Dutch; Reason 3: Not related to oral therapy-induced mucositis; Reason 4: Not related to pre-, pro-, post- or symbiotics; Reason 5: not in-vivo trial simulating oral mucositis
Data Extraction
Data was independently extracted by two reviewers (LF and IM) using a standardized table. In case of inconsistencies in the data collection process, a third author would resolve it through discussion. The following parameters were retrieved from each primary study: author, year, country, dates of information collection, study type, population characteristics (number of cases, type of treatment, age, control group), study design (such as type of administration and sampling time), biotics characteristics (such as designation and concentration), clinical outcomes, and main conclusions.
Risk of Bias Assessment
The Cochrane Collaboration tool was used to assess the risk of bias (“RoB”) for randomized controlled trials. The RoB evaluation was conducted separately by two reviewers (LF and IM) and classified as “high risk of bias”, “low risk of bias”, or “unclear risk of bias” if there is any incomplete or unclear data. In case of any inconsistency in the RoB assessment, a third author solved it through discussion (MJA). No RoB assessment was performed on observational before-after studies due to a lack of consensually accepted tools for assessing RoB in those specific studies.
Results
Study Characteristics
From a total of 9 studies, 6 were performed on humans [14,15,16,17,18,19], including 4 randomized controlled clinical trials [15, 17,18,19], while 3 were performed on animals [20,21,22]. The countries of origin of the studies were located in Asia [14, 16,17,18,19,20, 22] and Europe [15, 18, 21].
The pre-clinical studies used hamsters and mice which were experimentally irradiated [20, 21] or injected with 5-fluorouracil (5-FU) [22]. Regarding the control groups, these studies used a placebo (cryoprotectants and excipients of the formula) [21], saline lavage [20], or drinking water [22]. As for the biotics, only probiotics were tested. One article used a single probiotic Streptococcus salivarius K12 [20] and two articles used a combination of probiotics: (i) Caluwaerts et al. [21] used Lactococcus lactis sAGX0085 genetically modified to carry erythromycin (Em) and chloramphenicol (Cm) resistance genes and to secrete human Trefoil Factor 1 (htff1) (Lactococcus lactis sAGX0085Em + Cm + + htff1); and (ii) Gupta et al. [22] tested Lactobacillus reuteri DSM 17,938 and ATCC PTA 5289 strains. Regarding the methods used for administration, a topical application was used in two studies [20, 21] and in one study the probiotic was added to the drinking water [22]. The doses were given in a different posology, as displayed in Table 1. The three articles used macroscopic [20], histologic [20, 22], microbiologic [20], RNA analysis [22], qPCR analysis [22], cell culture [22] and/or immunohistochemistry [21] methods to determine the effect of probiotic use.
Among the 6 human studies included, there were a total of 381 children [14, 16] and adults [15,16,17,18,19] submitted to cancer treatment (207 participants in the intervention group and 174 in the control group), as displayed in Table 2. Participants were submitted to a wide range of oncological treatments that led to the development of OM: chemo-radiotherapy-induced OM was included in two studies [15, 17], chemotherapy-induced OM was reported in two studies [14, 18], chemotherapy combined with hematopoietic stem cell transplantation (HSCT) in one study [16], and in another study, patients were subjected to intensity modulated radiation therapy (IMRT) or concurrent chemo-radiotherapy with cisplatin [19]. These studies used as controls an oral lavage with bicarbonate [15] or a sodium chloride (NaCl) mouthwash [18], a benzidine hydrochloride mouth rinse along with baking soda or distilled water [19], and placebo lozenges (mixture of sugars and salts used as excipients in the active formulation) [17]. In two studies [14, 16], the authors had no control groups and compared the obtained results to other studies [14, 16]. All studies assessed how probiotics affected the severity of OM before and after probiotic intake. No studies assessing pre-, post-, or symbiotics were found. To evaluate the progression of this disease, one article [14] used the Oral Assessment Guide (15), two papers [15, 23] used the Common Terminology Criteria for Adverse Events (CTCAE) 4.0, and the other three [16,17,18] used the National Cancer Institute’s CTC (NCI CTC) scale. Regarding probiotic administration, in three studies [15,16,17], Lactobacillus brevis CD2 was administered 6 times per day, to be dissolved in the mouth and then swallowed [15,16,17]. In the other three studies, patients were instructed either to gargle with a mouthwash containing Lactobacillus casei and other Lactobacillus species (not specified) [14], either to ingest fermented food enriched in probiotics (e.g., kefir) [18], or to ingest 5 ml of an oral suspension containing about 2 million spores of Bacillus clausii UBBC − 07, combined with benzidine hydrochloride mouth rinse along with baking soda, twice a day [19].
Risk of Bias Within Studies
The risk of bias (RoB) was assessed to the four clinical trials retrieved, as displayed in Table 3. One randomized clinical trial (RCT) was marked as having a low RoB [17], while other two were marked as unclear [18, 19] and one having a high RoB [15]. The study of Christian et al. [14], although being designed as a clinical trial, did not have a control group, and cannot be considered an RCT. In the other studies, two uncontrolled before-after studies with a low number of participants [14, 16] and three studies in animals [20,21,22], the assessment of RoB was not feasible.
Results of Individual Studies
All the included pre-clinical studies [20,21,22] described probiotic interventions as effective in reducing OM severity. Four included studies in humans [14, 16, 17, 19] described that probiotic intervention was effective in reducing and preventing the degree and severity of oral mucositis in patients undergoing cancer therapy either radiotherapy, hematopoietic stem cell transplantation, or chemotherapy. Meanwhile, two studies reported that the difference in the incidence of oral mucositis between the intervention and control groups was not significant [15, 18].
Concerning the pre-clinical studies, Caluwaerts et al. [21] found that a mouth rinse containing 108 or 1010 CFU/dose of Lactococcus lactis sAGX0085Em + Cm + + htff1 (coded AG013) significantly reduced the period of severe OM in hamsters. It is noteworthy that AG013 was qualified as more effective than a mouth rinse or an oral spray containing high amounts of the therapeutic peptide itself because of the longer-lasting contact with the mucosa. In addition, the authors found that in single- and multiple-dose pharmacokinetic (PK) studies in healthy and irradiated hamsters, living and metabolic active AG-X0085Em + Cm + bacteria could be recovered from the oral cavity up to 24 h post-dosing, but there was no exposure beyond the mucosal compartment. These findings supported that the administration of AG013 to OM patients at risk of developing neutropenia is safe.
Wang et al. [20] stated that topical application of S. salivarius K12 significantly reduced the severity of OM in mice, finding that the relative area of mucositis including ulcers was significantly reduced in the intervention group (p < 0.001) and described the capacity of S. salivarius K12 in modulating the oral microbiome through the inhibition of oral anaerobes (reduced Pasteurella, Corynebacterium, Porphyromonas, and Staphylococcus). Moreover, in this study it was observed that in the group of irradiated (IR) mice treated with S. salivarius K12, the relative area of mucositis (including ulcers) was lower (9.03%) compared to the IR mice treated with a saline solution (77.42%), and had restored the integrity of the lingual mucosa, showing a thicker mucosa and basal layer epithelial cellularity. Finally, the weight of mice who received irradiation decreased sharply (-12,05 g), while S. salivarius K12 treatment lessened the body weight loss (-8.33 g).
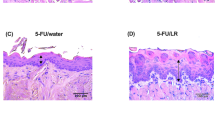
Gupta et al. [22] stated that the tested L. reuteri strains (LR) were effective in reducing OM severity, as it was found that the epithelial damage was less severe in the group injected with 5-FU and fed with LR in drinking water (5-FU/LR group) (p < 0.001) and had higher expression of Ki-67 protein (proliferation marker) in basal epithelial cells (p < 0.001) resulting in a higher epithelial regeneration, comparing to the 5-FU/water group. Additionally, it was shown that L. reuteri reduced oxidative stress through the nuclear factor E2-related factor-2 (Nrf-2) signaling. Concerning the safety of these strains, the probiotic administration did not result in systemic bacterial translocation, suggesting that these L. reuteri strains are safe for administration during chemotherapy.
Regarding the included clinical studies, Sharma, Tilak et al. [16] reported that the use of L. brevis was safe and effective in preventing OM in patients undergoing high-dose chemotherapy and autologous stem cell transplant (HSCT). L. brevis was administered to all participants as there was no control group. Fluconazole and itraconazole prophylaxis were given to all patients, and acyclovir prophylaxis to transplant patients. The results showed that around 19.4% of the patients developed severe OM, 58.1% of the patients presented mild to moderate OM, and a total of 22.5% of patients did not develop OM. The time to onset OM was 6 days and for resolution/healing, it took 8 days after the day of stem cell infusion.
Sharma, Rath et al. [17] stated that administration of Lactobacillus brevis CD2 lozenges in head and neck squamous cell carcinoma (HNSCC) patients enduring radiotherapy and concurrent cisplatin-based chemotherapy, reduced the incidence of severe OM (52% incidence in the intervention group versus 77% in the placebo group). It was also observed that the administration of this probiotic was able to reduce the occurrence of OM, as there were more remaining free OM patients in the intervention arm (28% vs. 7%). Regarding OM severity, 28% of the patients in the study arm did not develop OM, 19% developed mild to moderate mucositis, and 52% developed severe OM. On the other hand, 7% of the patients in the placebo arm did not develop OM, 15% developed mild to moderate mucositis (p < 0.001), and 77% developed severe OM (p < 0.001). The median time to the onset of mucositis was higher in the intervention group (22 days) than in the control group (18 days). However, the median time to heal mucositis was 43 days in both groups. It was also mentioned that no serious adverse events were observed when using L. brevis CD2 probiotic. Additionally, a higher percentage of patients in the L. brevis CD2 group (p = 0.001) completed the planned treatment (92% vs. 77%), not showing evidence of grade II nausea and vomiting and no non-compliance to the cancer treatment. Although there was a trend towards improvement in QOL in the L. brevis CD2 arm compared to the placebo, it was not statistically significant. However, it was also observed that, compared to the placebo group, with the use of L. brevis CD2, fewer patients required analgesics for mucositis-associated pain (p = 0.02). Moreover, among patients who were able to complete the anticancer treatment, the requirement for parenteral nutrition or Ryle’s tube insertion trended lower in the L. brevis CD2 arm.
Conversely, De Sanctis et al. [15] found that L. brevis CD2 had no effect in reducing the incidence of severe OM induced by intensity-modulated radiation therapy (IMRT) and concomitant cisplatin-based chemotherapy. De Sanctis et al. [15] reported that 40.6% of the patients in the intervention group and 41.6% of the patients with sodium bicarbonate mouthwash (control group) developed severe OM. It was also noticed that there was a statistically significant tendency for weight loss during concurrent therapy compared to baseline (p < 0.01), independently of the intervention or control arm. It was also noted that dysphagia was greater in the intervention arm (p < 0.05) and there was no difference between groups regarding pain evolution. Although probiotics were considered ineffective in reducing or preventing OM, it was stated that there was no serious adverse event related to L. brevis CD2 lozenge administration.
Christian et al. [14] concluded that there was a statistical difference (p < 0.05) in Oral Assessment Guide (OAG) before and after gargling with probiotics containing Lactobacillus species (not specified) and Lactobacillus casei in children with leukemia submitted to chemotherapy. It is noteworthy that there was a statistically significant decrease in OAG score between days 7 and 14 after gargling with the probiotics. Therefore, they concluded that probiotics could be an alternative therapy and prevention for oral mucositis.
According to Mirza et al. [19], taking Bacillus clausii UBBC − 07 twice a day allowed a substantial increase in the median time of mucositis onset (10 days in the intervention group versus 8 days in the control group; p < 0.01) and a significant decrease in the median time for remission (12 days in test and 14 days in control groups; p < 0.05). Additionally, it was described that, in the intervention group, 8 out of 23 patients had a significantly lower incidence of higher-grade OM (grade III or higher) compared to the control group (16 out of 23 patients; p < 0.05). In contrast to the placebo group, the test group did not experience diarrhea as a side effect of RT. Additionally, no adverse events associated with Bacillus clausii were observed.
Lastly, Topuz et al. [18] reported that kefir use was considered ineffective in decreasing OM severity. In fact, during chemotherapy, mucositis incidence increased significantly with increasing chemotherapy cycles in the kefir group (p = 0.009). However, this was not the case for the control group receiving an oral lavage with 0.09% NaCl (p = 0.29). When the two were compared for incidence of OM during therapy, no statistical significance was detected, as 72.7% of the intervention group did not develop OM (versus 78.3% of the patients in the control group). Additionally, the authors found that during chemotherapy, serum proinflammatory cytokines did not change significantly.
Discussion
Despite advances in medical therapy, the current knowledge in the area of prevention and treatment of therapy-induced oral mucositis is very limited. As previously stated, the management of oral mucositis remains mostly symptomatic and there is no effective strategy for its prevention or treatment [24]. Thus, there is a need to find new alternatives or complementary therapies. Consequently, knowing the positive effect of biotics in other diseases, and that some bacteria strains can modulate the epithelial cells, barrier function, mucosal immunity, and macrophage signaling pathways influencing cytokine production, we may consider biotics as a therapeutic possibility [24, 25]. As the occurrence of oral mucositis seems likely to happen after cancer therapy, the primary concern is to prevent its onset and progression. Probiotics have successfully been used to prevent and reduce mucositis severity in clinical and preclinical studies, but no studies were found using pre-, post- and symbiotics. Lactobacillus species intake, specifically L. brevis CD2 [16, 17], L. casei [14], and L. reuteri [22], as well as Streptococcus salivarius K12 [20], Bacillus clausiI UBBC − 07 spores [19] and Lactococcus lactis (AG013) [21] appear to be associated with a decrease in OM incidence and severity.
There are several mechanisms described to explain the effectiveness of these probiotic strains. As for Lactobacillus spp., these strains presented promising results and seemed to activate important anti-inflammatory mechanisms, which would benefit OM patients. For instance, Sharma, Rath et al. [17] explained that L. brevis CD2 produces high levels of arginine deiminase and sphingomyelinase which compete with nitric oxide synthase, leading to a reduction in the levels of some of the inflammatory factors (cytokines interleukin (IL)-1a, IL-6, IL-8, tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFNγ), prostaglandin E2 (PGE2) and matrix metalloproteinases). Furthermore, bacterial sphingomyelinase can hydrolyze the platelet-activating factor (PAF), a potent inflammatory cytokine, and is known to be associated with oral mucositis in radiation therapy [17]. Lee et al. [26] reported that Lactobacillus casei significantly decreased TNF-α, and IL-6, and adhered to surface molecules by suppressing the signaling pathway of IL-6 and TNF-α. Amdekar et al. [27] mentioned that L. casei induces ciclo-oxigenase-2 (COX-2) inhibition, having an antiarthritic effect. Lastly, Gupta et al. [22] reported that Lactobacillus reuteri DSM 17,938 and ATCC PTA 5289 strains seem to be capable of modulating the host inflammatory response by reducing pro-inflammatory cytokine response (e.g., TNF-α, IL-beta, and Myeloperoxidase (MPO)) and of increasing key antioxidant genes (i.e., superoxide dismutase-1 (SOD-1), glutathione peroxidase-1 (GPx-1), and heme oxygenase-1 (HO-1)). In recent literature, Lactobacillus reuteri has been associated with reduced gingival inflammation and a decrease in pathogens associated with periodontitis [28]. Mu et al. [29] demonstrated that L. reuteri can produce antimicrobial molecules, such as organic acids, ethanol, and reutein, inhibiting the colonization of pathogenic microbes and remodeling commensal microbiota. The immunomodulatory effects of the probiotic Bacillus clausii in intestinal health are well established, as well as its ability to treat gastrointestinal discomfort. However, there may be further advantages in other therapeutic fields that are only now being identified [30]. For instance, according to Nirmala et al. [31], using B. clausii as a local adjuvant greatly decreases the symptoms of oral candidiasis and recurrent aphthous ulcers. Wang et al. [20] mentioned that S. salivarius K12 can modulate the oral microbiome, reducing the abundance of anaerobic bacteria and ulceration, increasing the thickness of the tongue mucosa and the density of basal cells, and enhancing basal cell proliferation and attenuating apoptosis. Burton et al. [32] showed in vitro that S. salivarius K12 suppressed the growth of different strains of bacteria implicated in halitosis, enhancing the capacity to modulate the microbial ecosystem. Caluwerts et al. [21], genetically modified Lactococcus lactis strain sAGX0085 engineered to secrete human Trefoil Factor 1. The authors stated that TFF1 was found as a gastric tumor suppressor and, at the cellular level, TFF1 promotes cell differentiation while limiting cell proliferation and apoptosis [21]. Strains of Lactococcus lactis have only recently been explored for their possible cytotoxic effects against human cancer cell lines [33] and anti-inflammatory properties and capacity in preventing 5-FU-induced gut dysbiosis [34]. In summary, the anti-inflammatory, immunomodulatory, and antioxidant properties of probiotic strains would be of great value considering the five-step pathogenesis model of OM proposed by Sonis [3], as they may protect against the negative effects of radiotherapy and chemotherapy on the oral mucosa.
When evaluating the effectiveness of a probiotic, it is essential to understand whether there is a trend towards the improvement of the QOL. The WHO defines the QOL as an individual’s perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards, and concerns. Regarding the QOL, the results between pre-clinical and clinical studies differed. In other diseases, some studies reported an improvement in the QOL of individuals who were given probiotics. This is the case of Waal et al. [33], in which the authors found an improvement in QOL in 66% of the patients suffering from ulcerative colitis taking a probiotic. Nevertheless, more studies are needed to evaluate the impact of probiotic intake in the QOL of OM patients.
Considering that all cancer patients are considered immune-depressed and some HNC patients develop neutropenia, the evaluation of the safety of probiotic strains is essential. Although most of the above-mentioned studies reported that probiotics are safe, the Agency for Healthcare Research and Quality (AHRQ) [35], in 2011, concluded that there is still a lack of evidence to confidently recommend probiotic interventions to the healthcare and nutrition communities. According to the World Health Organization in 2002, probiotics may theoretically be responsible for systemic infections, deleterious metabolic activities, excessive immune stimulation in susceptible individuals, and gene transfer [36]. Nevertheless, the AHRQ [35] also affirmed that the lack of adverse events supports the safety of probiotics. The European Food Safety Authority (EFSA) has already recognized the health benefits that are pertinent to the effects of probiotics in some health conditions in 2021, under the Nutrition & Health Claims Regulation [37]. However, EFSA has not yet published guidelines concerning the safety of probiotic use, specifically in immune-depressed patients [37]. As such, further studies are necessary to evaluate the safety concerns of probiotic treatment in immunocompromised patients.
Although our findings support the conclusion that probiotics may reduce the onset and severity of cancer therapy-induced OM, some potential limitations should be addressed. Firstly, the number of studies examined was small (n = 9) and with heterogeneous study designs. For example, the difference in findings between the studies from De Sanctis et al. [15], Sharma, Tilak et al. [16], and Sharma et al. [17], where authors tested L. brevis strains but only the two studies of Sharma et al. [16, 17] showed positive effects on oral mucositis. These outcomes could be explained by different cancer treatments, and different control groups (sodium bicarbonate mouthwash versus placebo lozenges, respectively) which could induce a lower rate of severe OM. Referring to the cancer treatment, it is important to state that IMRT may have improved tolerance to concomitant chemoradiotherapy (RCHT) or intraoperative radiotherapy (bioRT), reducing the effectiveness of L. brevis CD2. Secondly, cancer type and treatment differed across studies. For instance, Sharma, Rath et al. [17] included patients receiving radical radiotherapy at a dose of 70 Gy and chemotherapy of cisplatin, while Sharma, Tilak et al. [16] included patients in a chemotherapy regime with hematopoietic stem cell transplantation (HSCT). Third, the probiotic composition, posology, mode of administration, and additional treatments varied across studies. For example, Topuz et al. [18] considered the use of kefir ineffective in decreasing OM severity. However, kefir had a short permanence in the oral cavity since it was ingested, while in other studies the probiotics were dissolved in the mouth before ingesting [15,16,17] or applied as a mouthwash for a defined period [14] or applied as an oral suspension [19], resulting in a long-lasting direct contact with the oral cavity. Thus, the mode of administration and the time of contact could influence the impact of the probiotic on the progression of OM. It should also be noted that some studies used sodium chloride or sodium bicarbonate in the control group [19]. Both sodium bicarbonate and sodium chloride are known to be effective in treating and reducing the severity of oral mucositis [38] and promoting healthy gum and improving oral ulcer healing [39], respectively. The use of these components could influence the results due to their influence on oral physiology. It should also be noted that the quality of the studies is overall low because there are unclear aspects and only one study [17] was considered to have a low risk of bias. To summarize, probiotics appear to be a safe treatment option for cancer therapy-induced OM, but additional research is needed to assure their efficacy and security as well as to better define the most efficient posology and formulation. Moreover, would be also relevant to explore other biotics formulations, such as pre-, post-, and symbiotics.
Conclusions
In conclusion, the findings of this systematic review suggest that probiotic supplementation could potentially reduce the incidence of therapy-induced oral mucositis or alleviate its symptoms in chemotherapy or radiotherapy patients. The available evidence, however, is limited and marred by significant heterogeneity across studies. Taking these findings into account, we suggest further research particularly regarding the probiotic strains of L. brevis CD2, L. reuteri, L. casei, S. salivarius K12, B. clausii UBBC − 07 spores, and Lactococcus lactis (AG013), as these presented promising results. Further recommendations for future studies include the use of probiotic combinations, bearing in mind possible beneficial interactions, as well as standardized control groups. It is also critical to determine the proper probiotic posology and formulation for better results and safety and to develop guidelines for safe probiotic use, particularly in immunocompromised patients.
Data Availability
Not applicable.
References
Seiler S, Kosse J, Loibl S, Jackisch C (2014) Adverse event management of oral mucositis in patients with breast cancer. Breast Care 9(4). https://doi.org/10.1159/000366246
Kwon Y (2016) Mechanism-based management for mucositis: option for treating side effects without compromising the efficacy of cancer therapy. Onco Targets Ther 9. https://doi.org/10.2147/OTT.S96899
S.T. S. Pathobiology of oral mucositis: novel insights and opportunities. J Supportive Oncol. ;5(9 SUPPL. 4).
Hou J, Zheng HM, Li P, Liu HY, Zhou HW, Yang XJ (2018) Distinct shifts in the oral microbiota are associated with the progression and aggravation of mucositis during radiotherapy. Radiother Oncol 129(1). https://doi.org/10.1016/j.radonc.2018.04.023
Lalla R, Saunders DP, Peterson DE (2014) Chemotherapy or Radiation-Induced oral mucositis. Dent Clin North Am 58(2). https://doi.org/10.1016/j.cden.2013.12.005
Brown TJ, Gupta A (2020) Management of cancer therapy-associated oral mucositis. J Oncol Pract 16(3). https://doi.org/10.1200/JOP.19.00652
Degruttola AK, Low D, Mizoguchi A, Mizoguchi E (2016) Current understanding of dysbiosis in disease in human and animal models. Inflamm Bowel Dis 22(5). https://doi.org/10.1097/MIB.0000000000000750
Xu Y, Teng F, Huang S et al (2014) Changes of saliva microbiota in nasopharyngeal carcinoma patients under chemoradiation therapy. Arch Oral Biol 59(2). https://doi.org/10.1016/j.archoralbio.2013.10.011
Elad S, Cheng KKF, Lalla R et al (2020) MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 126(19). https://doi.org/10.1002/cncr.33100
E C, M C, R C. Probiotics and mucositis. Curr Opin Clin Nutr Metab Care. ;21(5)
Salminen S, Collado MC, Endo A et al (2021) The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol 18(9). https://doi.org/10.1038/s41575-021-00440-6
Szajewska H, Mrukowicz JZ (2001) Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: a systematic review of published randomized, double-blind, placebo-controlled trials. J Pediatr Gastroenterol Nutr 33. https://doi.org/10.1097/00005176-200110002-00004
Matsubara VH, Bandara HMHN, Ishikawa KH, Mayer MPA, Samaranayake LP (2016) The role of probiotic bacteria in managing periodontal disease: a systematic review. Expert Rev Anti Infect Ther 14(7). https://doi.org/10.1080/14787210.2016.1194198
Christian H, Suharsini M, Fauziah E (2020) Effects of probiotics on clinical appearance of oral mucositis in children with leukemia during chemotherapy. Int J Dent Oral Sci 7(11). https://doi.org/10.19070/2377-8075-20000204
de Sanctis V, Belgioia L, Cante D et al (2019) Lactobacillus brevis CD2 for Prevention of oral Mucositis in patients with Head and Neck Tumors: a multicentric randomized study. Anticancer Res 39(4). https://doi.org/10.21873/anticanres.13303
Sharma A, Tilak T, Bakhshi S et al (2016) Lactobacillus brevis CD2 lozenges prevent oral mucositis in patients undergoing high dose chemotherapy followed by haematopoietic stem cell transplantation. ESMO Open 1. https://doi.org/10.1136/esmoopen-2016-000138
Sharma A, Rath GK, Chaudhary SP, Thakar A, Mohanti BK, Bahadur S (2012) Lactobacillus brevis CD2 lozenges reduce radiation- and chemotherapy-induced mucositis in patients with head and neck cancer: a randomized double-blind placebo-controlled study. Eur J Cancer 48(6). https://doi.org/10.1016/j.ejca.2011.06.010
Topuz E, Derin D, Can G et al (2008) Effect of oral administration of kefir on serum proinflammatory cytokines on 5-FU induced oral mucositis in patients with colorectal cancer. Invest New Drugs 26(6). https://doi.org/10.1007/s10637-008-9171-y
Mirza MA, Aruna D, Irukulla M (2022) Efficacy of Bacillus clausii UBBC – 07 spores in the amelioration of oral mucositis in head and neck cancer patients undergoing radiation therapy. Cancer Treat Res Commun 31. https://doi.org/10.1016/j.ctarc.2022.100523
Wang Y, Li J, Zhang H et al (2021) Probiotic Streptococcus salivarius K12 alleviates Radiation-Induced oral mucositis in mice. Front Immunol 12. https://doi.org/10.3389/fimmu.2021.684824
Caluwaerts S, Vandenbroucke K, Steidler L et al (2010) AG013, a mouth rinse formulation of Lactococcus lactis secreting human Trefoil factor 1, provides a safe and efficacious therapeutic tool for treating oral mucositis. Oral Oncol 46(7). https://doi.org/10.1016/j.oraloncology.2010.04.008
Gupta N, Ferreira J, Hong CHL, Tan KS (2020) Lactobacillus reuteri DSM 17938 and ATCC PTA 5289 ameliorates chemotherapy-induced oral mucositis. Sci Rep 10(1). https://doi.org/10.1038/s41598-020-73292-w
Mirza MA, Aruna D, Irukulla M (2022) Efficacy of Bacillus clausii UBBC – 07 spores in the amelioration of oral mucositis in head and neck cancer patients undergoing radiation therapy. Cancer Treat Res Commun 31. https://doi.org/10.1016/j.ctarc.2022.100523
Llewellyn A, Foey A (2017) Probiotic modulation of innate cell pathogen sensing and signaling events. Nutrients 9(10). https://doi.org/10.3390/nu9101156
Cristofori F, Dargenio VN, Dargenio C, Miniello VL, Barone M, Francavilla R (2021) Anti-inflammatory and Immunomodulatory Effects of Probiotics in Gut inflammation: a door to the body. Front Immunol 12. https://doi.org/10.3389/fimmu.2021.578386
Lee JM, Hwang KT, Jun WJ, Park CS, Lee MY (2008) Antiinflammatory effect of lactic acid bacteria: inhibition of cyclooxygenase-2 by suppressing nuclear factor-κB in Raw264.7 macrophage cells. J Microbiol Biotechnol. ;18(10)
Amdekar S, Singh V, Singh R, Sharma P, Keshav P, Kumar A (2011) Lactobacillus casei reduces the inflammatory joint damage associated with collagen-induced arthritis (CIA) by reducing the pro-inflammatory cytokines - Lactobacillus casei: COX-2 inhibitor. J Clin Immunol 31(2). https://doi.org/10.1007/s10875-010-9457-7
Szkaradkiewicz AK, Stopa J, Karpiński TM (2014) Effect of oral administration involving a probiotic strain of Lactobacillus reuteri on pro-inflammatory cytokine response in patients with chronic Periodontitis. Arch Immunol Ther Exp (Warsz) 62(6). https://doi.org/10.1007/s00005-014-0277-y
Mu Q, Tavella VJ, Luo XM (2018) Role of Lactobacillus reuteri in human health and diseases. Front Microbiol 9(APR). https://doi.org/10.3389/fmicb.2018.00757
Ghelardi E, Abreu Y, Abreu AT, Marzet CB, Calatayud G, Perez MIII, Castro APM (2022) Current progress and future perspectives on the use of Bacillus clausii. Microorganisms 10(6). https://doi.org/10.3390/microorganisms10061246
Nirmala M, Smitha SG, Kamath GJ (2019) A study to assess the efficacy of local application of oral probiotic in treating recurrent aphthous ulcer and oral candidiasis. Indian J Otolaryngol Head Neck Surg 71. https://doi.org/10.1007/s12070-017-1139-9
Burton JP, Chilcott CN, Moore CJ, Speiser G, Tagg JR (2006) A preliminary study of the effect of probiotic Streptococcus salivarius K12 on oral malodour parameters. J Appl Microbiol 100(4). https://doi.org/10.1111/j.1365-2672.2006.02837.x
van der Waal MB, Flach J, Browne PD, Besseling-van der Vaart I, Claassen E, van de Burgwal LHM (2019) Probiotics for improving quality of life in ulcerative colitis: exploring the patient perspective. PharmaNutrition 7. https://doi.org/10.1016/j.phanu.2018.100139
Carvalho R, Vaz A, Pereira FL et al (2018) Gut microbiome modulation during treatment of mucositis with the dairy bacterium Lactococcus lactis and recombinant strain secreting human antimicrobial PAP. Sci Rep 8(1). https://doi.org/10.1038/s41598-018-33469-w
Wallace TC, MacKay D (2011) The safety of probiotics: considerations following the 2011 U.S. agency for health research and quality report. J Nutr 141(11). https://doi.org/10.3945/jn.111.147629
FAO/WHO. Guidelines for the evaluation of probiotics in food. Food and Agriculture Organization of the United Nations and World Health Organization Group Report.(London Ontario, Canada). FAO Food and Nutrition Paper 85. Published online 2002
Food Supplements Europe (2021) Probiotics: Growing Science and Need for Proper Consumer Communication on Probiotic Food Supplements.;
Mohammadi F, Oshvandi K, Kamallan SR et al (2022) Effectiveness of sodium bicarbonate and zinc chloride mouthwashes in the treatment of oral mucositis and quality of life in patients with cancer under chemotherapy. Nurs Open 9(3). https://doi.org/10.1002/nop2.1168
Huynh NCN, Everts V, Leethanakul C, Pavasant P, Ampornaramveth RS (2016) Rinsing with saline promotes human gingival fibroblast wound healing in vitro. PLoS ONE 11(7). https://doi.org/10.1371/journal.pone.0159843
Funding
Open access funding provided by FCT|FCCN (b-on). M.J.A.’s Ph.D. fellowship was supported by an FCT scholarship (SFRH/BD/144982/2019).
Author information
Authors and Affiliations
Contributions
Leonor Furtado, Inês Magalhães, Maria João Azevedo, and Benedita Sampaio-Maia were responsible for the study design. Data was acquired, analyzed, and interpreted by Leonor Furtado, Inês Magalhães, and Maria João Azevedo. The manuscript was prepared by Leonor Furtado. All authors contributed to the editing and reviewing of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Conflit of Interest
None declared.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Frey-Furtado, L., Magalhães, I., Azevedo, M.J. et al. The Role of Biotics as a Therapeutic Strategy for Oral Mucositis - A Systematic Review. Probiotics & Antimicro. Prot. (2023). https://doi.org/10.1007/s12602-023-10116-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s12602-023-10116-z