Abstract
Heart failure (HF) is a global pandemic with increasing prevalence and mortality rates annually. Its main cause is myocardial infarction (MI), followed by rapid cardiac remodeling. Several clinical studies have shown that probiotics can improve the quality of life and reduce cardiovascular risk factors. This systematic review and meta-analysis aimed to investigate the effectiveness of probiotics in preventing HF caused by a MI according to a prospectively registered protocol (PROSPERO: CRD42023388870). Four independent evaluators independently extracted the data using predefined extraction forms and evaluated the eligibility and accuracy of the studies. A total of six studies consisting of 366 participants were included in the systematic review. Probiotics are not significant in intervening left ventricular ejection fraction (LVEF) and high-sensitivity C-reactive protein (hs-CRP) when compared between the intervention group and the control group due to inadequate studies supporting its efficacy. Among sarcopenia indexes, hand grip strength (HGS) showed robust correlations with the Wnt biomarkers (p < 0.05), improved short physical performance battery (SPPB) scores were also strongly correlated with Dickkopf-related protein (Dkk)-3, followed by Dkk-1, and sterol regulatory element-binding protein 1 (SREBP-1) (p < 0.05). The probiotic group showed improvement in total cholesterol (p = 0.01) and uric acid (p = 0.014) compared to the baseline. Finally, probiotic supplements may be an anti-inflammatory, antioxidant, metabolic, and intestinal microbiota modulator in cardiac remodeling conditions. Probiotics have great potential to attenuate cardiac remodeling in HF or post-MI patients while also enhancing the Wnt signaling pathway which can improve sarcopenia under such conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure (HF) has become a global pandemic with increasing prevalence and mortality rates every year [1]. The main cause of HF is myocardial infarction (MI) which is followed by a rapid process of cardiac remodeling [2]. Besides that, disturbances in metabolic and inflammatory pathways are suspected of helping accelerate the development and progression of HF [3]. In addition, to poor prognosis HF unfortunately has a high health expenditure burden [4]. Furthermore, patients with HF experience significant functional impairment in daily activities due to muscle atrophy, weakness, and reduced endurance capacity [1, 4].
Some of the available standard management of MI treatment such as antiplatelet therapy and percutaneous coronary intervention has not been able to prevent the development of MI into HF [5]. Several breakthroughs have been made to reduce HF due to MI, such as administering angiotensin-converting enzyme (ACE) inhibitors, β-receptor blocking agents, and aldosterone receptor antagonists, but the prevalence of HF in MI patients is still high [5, 6]. In addition, this breakthrough has not been able to improve the quality of life (QoL) of MI patients [6].
Dysbiosis is a condition in which there is an alteration of the gut microbiota [7], and this condition is suspected of causing elevated levels of reactive oxygen species, lipopolysaccharides (LPSs), and harmful metabolites that can lead to cardiac hypertrophy, fibrosis, and an increase in proinflammatory cytokines, which are the primary risk factors of cardiovascular disease (CVD) [8]. According to recent investigations, probiotics offer a promising therapy to reduce inflammation, permeability, and translocation of LPSs and harmful metabolites into circulation, and oxidative stress, thereby reducing cardiac hypertrophy and contractile dysfunction in HF patients [9, 10]. In addition to reducing risk factors for cardiovascular disease, probiotics have the potential to increase patient QoL because probiotics can boost skeletal muscle mass [11].
To the best of our knowledge, no literature review regarding the effects of probiotics supplementation on attenuating cardiac remodeling following the MI is published or available. Therefore, we conducted a systematic review and meta-analysis to assess the effects of probiotics on preventing HF caused by MI.
Materials and Methods
Searches Strategy
Our main search for this review was randomized controlled trials (RCTs) or clinical trials that examined the impact of probiotics supplementation on reducing cardiac remodeling in the condition of HF or high risk for developing HF such as post-MI. A search for clinical trials other than RCTs was also carried out. We searched Embase, PubMed, the Cochrane Library, Wiley, and ProQuest before January 2, 2022. The search was performed using the Boolean operator method and used the following keywords: (probiotics OR synbiotics OR prebiotics) AND (cardiac remodeling OR heart failure OR cardiomyopathy OR post-myocardial infarction).
Study Selection
Inclusion Criteria
-
1.
Randomized controlled trials and clinical trials which were conducted and published within the last 10 years were used for the investigation.
-
2.
A sample population of patients with HF fulfilling the diagnosis criteria or post-MI patients to assess the feasibility of the proposed intervention.
-
3.
Peer-reviewed journals.
-
4.
Studies using probiotic supplementation as its intervention.
Exclusion Standards
-
1.
Patients with comorbidities or other cardiovascular disorders.
-
2.
Patients with probiotic or standard treatment allergies.
-
3.
Non-human trials and studies.
-
4.
Clinical trials using a crossover study design.
-
5.
Studies that are lacking essential outcome indices.
Data Extraction and Outcome Measures
Four independent evaluators independently extracted the data using predefined extraction forms in a Google Sheet. Each author evaluated the eligibility and accuracy of the studies. Discussions were used to settle any disputes on the studies during the authoring phase. Each study’s data extraction yielded the following items: first author, reference, publication year, country of study, study design, sample size (male/female), age, center, participant status, dose, probiotics strain, time to follow-up, and the primary outcome (scale, baseline, post-treatment, and mean changes from baseline).
Main Outcome(s)
The mean deviation of biomarker values from the baseline for each trial was the subject of this review. Procollagen III, TGF-β, TMAO, matrix metallopeptidase 9 (MMP-9), and serum high-sensitivity C-reactive protein (hs-CRP) are the biomarker measures that are covered. The change in left ventricular ejection fraction (LVEF) measured by echocardiography was also analyzed as the reflection of myocardial contractility.
Measures of Effect
We investigated the standardized mean difference (SMD) between the probiotics intervention and the control group.
Additional Outcome(s)
The secondary result was observing each intervention’s adverse events and clinical symptoms.
Measures of Effect
The additional outcomes were reported descriptively as a percentage of both intervention and control groups.
Risk of Bias Assessment
Using the Cochrane “Risk of Bias” 2 assessment tool [12], the quality of the included studies was assessed. This tool or software examined the following domains: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. The four writers carried out the process while others performed as supervisors, taking into account their prior knowledge of the tool and their experience with it. To settle any disagreements, the authors collaborated and had discussions. The domains were divided into three groups based on their level of risk of bias: low, some concern, or high risk of bias.
Statistical Analysis
Review Manager Software (Version 5.4; Oxford, England) was used for statistical analysis. Categorical data were assessed employing odds ratio (OR) with a 95% confidence interval (CI) and continuous data were analyzed using mean difference (MD) with 95% CI. Heterogeneity among studies was assessed by the I-squared (I2) test. I2 ≤ 50% was considered low heterogeneity, and the fixed-effects model was adopted; otherwise, it was deemed significant heterogeneity, and the random-effects model was adopted. To avoid biases caused by methodological differences among studies, we used sensitivity analyses to find the source of heterogeneity and inconsistency. Full text was evaluated to find the research of the origins of heterogeneity and investigated its influence on meta-analysis.
Ethical Approval
Ethical approval and informed consent of patients were not needed for the review because the authors only collected data from previous studies that had been published with their respective ethical approval.
Protocol Register
This research scheme was conducted under the guidance of Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) 2020 protocols [13], and has also been reviewed by the boards of PROSPERO-NIHR (International Prospective Register of Systematic Reviews–National Institute for Health Research) and has been registered with the number CRD42023388870.
Results
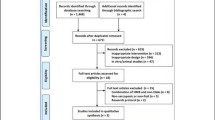
A total of six (6) RCTs were included in the systematic review, which yielded a total of 366 participants who were assessed using probiotics as the intervention and placebo as the control (Fig. 1).
Of all the included studies, three studies underwent quantitative analysis. All RCTs were also analyzed to determine the risk of bias in each study and narratively synthesize the results of each outcome reported (Fig. 2). The studies, which were published within the last 10 years, were done across various nations (Pakistan, Iran, and Brazil). The participants are post-MI or HF patients and are distributed almost equally in each gender (M/F). They were randomly assigned to an intervention group and a control group. Probiotic interventions came in a variety of probiotic strains and doses. Left ventricle ejection fraction (LVEF) and high-sensitivity C reactive protein (hs-CRP) were utilized to measure outcomes and further analyzed quantitatively. Table 1 includes a detailed list of the included studies’ characteristics.
Analysis for risk of bias. Six studies were analyzed for a variety of biases using the tools in RevMan software. A Traffic light plot of study quality assessment based on Cochrane RoB Tool 2.0. B Summary plot of study quality assessment based on Cochrane RoB 2.0. The risk for bias that was analyzed was evaluated using the Revised Tool RoB 2.0, which has five domains for studies. The results were recorded in the domain file bias (.xlsx) and then processed to the ROBVIS website for display and summary of the study
Left Ventricular Ejection Fraction (LVEF)
There were 3 studies that reported probiotics as an intervention vs placebo as a control with LVEF as their outcome measure (Fig. 3). The result was insignificant with p > 0.05 (p = 0.87) and MD value: − 0.20 (95% CI: − 2.48 to 2.09, I2 = 0%). However, the heterogeneity is insignificant. All things considered, it can be concluded that probiotics are not significant in intervening LVEF in the intervention group compared to the control group.
High-Sensitivity C-Reactive Protein (hs-CRP)
Probiotics were reported in 2 studies as an intervention versus placebo as the control in assessing hs-CRP (Fig. 4). With p > 0.05 (p = 0.06) and an MD value of − 0.34 (95% CI: − 0.69 to 0.01, I2 = 0%), the outcome was not statistically significant though the heterogeneity is insignificant. All things considered, it can be said that probiotics had no discernible effect on hs-CRP when compared between the intervention group and the control group due to inadequate studies supporting its efficacy. However, in a single study, it reduced hs-CRP significantly compared to the placebo [17]. In the other study, it reduced hs-CRP insignificantly, while the placebo group showed a significant increase in hs-CRP [19].
Biomarker Parameters
This systematic review included articles that reported different biomarkers. One of the biomarkers mentioned is zonulin, which serves as an indicator of intestinal permeability [14]. Other reported inflammatory biomarkers besides hs-CRP are CRP and sTWEAK [17, 19]. The reported oxidative stress biomarkers are 8-isoprostane and Ox-LDL [14]. Biomarkers in the Wnt signaling pathway which is correlated with sarcopenia were also reported in one study, including the Dkk-1, Dkk-3, and SREBP1 biomarkers [14]. In addition, various biomarkers were also reported, such as sCD163, ADMA, LCAT, BUN, pentraxin3, and ApoB100 [15]. Cardiac remodeling biomarkers include TGF-β, TMAO, MMP-9, procollagen III, and NT-proBNP (Table 2).
Probiotics exhibited an improvement effect in intestinal permeability by reducing zonulin. Probiotics also have anti-inflammatory properties as seen from a decrease in CRP and sTWEAK, which leads to a decrease in oxidative stress marked by a decrease in oxidative stress markers such as 8-isoprostane and Ox-LDL. In the end, probiotics reduce several cardiac remodeling triggers, including TGF-β and TMAO [17]. Probiotics also improved Wnt signaling in HF patients, which correlated with an improvement in sarcopenia.
Echocardiographic Parameters
Two studies reported echocardiographic parameters other than LVEF, including LVEDV, LVESV, and left atrial diameter. Probiotics appeared to improve LVEDV and LVESV but both measurements did not reach statistical significance [17]. In the other study, probiotics improved left atrial diameter by reducing significantly from baseline measurement [19].
Sarcopenia and Functional Capacity
One study featured sarcopenia indices and functional capacity outcomes, including hand grip strength (HGS), appendicular skeletal mass index (ASMI), fat mass, phase angle, gait speed, and short physical performance battery (SPPB) (Table 2). Compared to the baseline, there was an improvement in phase angle, HGS, gait speed, and SPPB. Among sarcopenia indexes, HGS showed robust correlations with the three Wnt biomarkers (p < 0.05). Improved SPPB scores were also strongly correlated with Dkk-3, followed by Dkk-1, and SREBP1 (p < 0.05).
Miscellaneous Laboratory and Microbiota Parameters
There is one trial that showed laboratory results in the form of blood sugar, total cholesterol, leukocyte count, creatinine, and uric acid changes after the intervention of probiotics and placebo [19]. The probiotic group showed improvement in total cholesterol (p = 0.01) and uric acid (p = 0.014) compared to the baseline, while the other parameters did not reach statistical significance in changes. Furthermore, one RCT analyzed the abundance of the butyrate-acetoacetate CoA transferase gene 3 months after intervention in the Norwegian study participants. No difference was observed in levels at 3 months between the control group and probiotic group (S. boulardii) (Table 2).
Discussion
Numerous research studies have shown mechanisms connecting the gut microbiota’s function in low-grade inflammation in cardiovascular disease (CVD) situations in recent years [20]. Numerous investigations have also revealed a connection between the development of HF and gut microbiota [21]. Alteration in gut barrier function (dysbiosis) also leads to increased TMAO levels [22]. It has been also found that TMAO levels are substantially higher in individuals with HF compared with that in control subjects [23] and additionally, TMAO-induced cardiac hypertrophy and cardiac fibrosis [24]. Furthermore, TMAO levels are strongly associated with gut microbiota and it has been found that gut microbiota modulation using probiotics can lead to a decrease in TMAO levels [25]. Still, the precise processes through which probiotics may influence the cardiac remodeling process are unknown. TMAO, short-chain fatty acids (SCFAs), and bile acids are examples of gut microbiota metabolites that may have an impact on the development of HF [26]. Kombucha probiotic drink from butterfly pea flower was shown to modulate gut microbiota and metabolic syndrome markers paired with considerable antioxidant and metabolite compounds [27]. Metabolic endotoxemia might benefit from probiotic use (Fig. 5) [28].
Additionally, the microbiota in HF patients may have a diminished ability to synthesize advantageous metabolites such SCFAs. Maintaining the mucosal barrier of the gut requires SCFAs, particularly butyrate. Loss of barrier function may make it easier for bacteria’s lipopolysaccharides to leak out (LPS). These substances may in turn trigger pattern recognition receptors in the innate immune system. These processes could be a factor in the low-grade systemic inflammation found in HF [29,30,31]. Fundamentally, SCFAs were proven to contribute beneficial effects to CVD [32]. The pathology of HF may be significantly influenced by SCFAs, possibly through an agonistic effect on G-protein-coupled receptors, inhibition of histone deacetylases (HDACs), restoration of mitochondrial function, amelioration of cardiac inflammatory response, use of SCFAs as an energy source, and distant effects attributed to a protective effect on other organs. Collectively, SCFAs may be an important mediator in the gut-heart axis in the pathophysiology of HF [33]. Two additional SCFAs, acetate and propionate, may also influence the renin-angiotensin system via G-protein-coupled olfactory receptors, establishing a link between the gut microbiota and the activation of neurohormonal pathways in HF. Additionally, acetate has been demonstrated in experimental investigations to lessen cardiac hypertrophy, lessen cardiac fibrosis, and enhance cardiac function [34,35,36].
This is the first systematic review and meta-analysis to examine the potential of probiotics in HF and cardiac remodeling (Fig. 5). All randomized controlled trials on the mentioned topics were included and analyzed quantitatively and narratively. Comprehensiveness and novelty are the main strengths of this systematic review. Nevertheless, there are several limitations to this systematic review. First, studies regarding the effects of probiotics on cardiac remodeling are still very heterogeneous in terms of outcomes and also the study population. This problem certainly arises from the very complex nature of the course of cardiac remodeling. This has an impact on the lack of certainty of evidence presented in this systematic review. Going forward, the provision of randomized clinical trials and updating of systematic reviews is needed to increase the certainty of evidence on this topic. Second, the available studies still use different kinds and doses of probiotics between studies. This problem makes the review results ambiguous and less specific. Lastly, the number of samples included in this analysis was relatively small given the short duration of follow-up or intervention to observe cardiac remodeling.
Conclusions
Probiotics supplementation can increase anti-inflammatory with anti-oxidant properties and accompanied by metabolic and gut modulation activities in the condition of cardiac remodeling. Therefore, it has a great potential to attenuate or prevent cardiac remodeling in HF or post-MI patients. Probiotics also enhance the Wnt signaling pathway which could improve sarcopenia under such conditions.
Data Availability
No data are associated with this article.
References
Kuno T, Tanimoto E, Morita S, Shimada YJ (2019) Effects of bariatric surgery on cardiovascular disease: a concise update of recent advances. Front Cardiovasc Med 6:94. https://doi.org/10.3389/fcvm.2019.00094
Leancă SA, Crișu D, Petriș AO, Afrăsânie I, Genes A, Costache AD et al (2022) Left ventricular remodeling after myocardial infarction: from physiopathology to treatment. Life 12:1111. https://doi.org/10.3390/life12081111
Kiluk M, Lewkowicz J, Pawlak D, Tankiewicz-Kwedlo A (2021) Crosstalk between tryptophan metabolism via kynurenine pathway and carbohydrate metabolism in the context of cardio-metabolic risk—review. J Clin Med 10:2484. https://doi.org/10.3390/jcm10112484
Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW et al (2021) Heart disease and stroke statistics - 2021 update: a report from the American Heart Association. Circulation 143:E254-743. https://doi.org/10.1161/CIR.0000000000000950
Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A et al (2018) 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur Heart J 39:213–254. https://doi.org/10.1093/eurheartj/ehx419
Bahit MC, Kochar A, Granger CB (2018) Post-myocardial infarction heart failure. JACC Hear Fail 6:179–186. https://doi.org/10.1016/j.jchf.2017.09.015
Lee J-Y, Tsolis RM, Bäumler AJ (2022) The microbiome and gut homeostasis. Science 377:eabp9960. https://doi.org/10.1126/science.abp9960
Kibel A, Lukinac AM, Dambic V, Juric I, Relatic KS (2020) Oxidative stress in ischemic heart disease. Oxid Med Cell Longev. https://doi.org/10.1155/2020/6627144
Cianci R, Franza L, Borriello R, Pagliari D, Gasbarrini A, Gambassi G (2022) The role of gut microbiota in heart failure: when friends become enemies. Biomedicines 10:2712. https://doi.org/10.3390/biomedicines10112712
Szabo TM, Frigy A, Nagy EE (2021) Targeting mediators of inflammation in heart failure: a short synthesis of experimental and clinical results. Int J Mol Sci 22. https://doi.org/10.3390/ijms222313053
Liu C-F, Tang WHW (2022) Gut microbiota in sarcopenia and heart failure. J Cardiovasc Aging 2:35. https://doi.org/10.20517/jca.2022.07
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:1–8. https://doi.org/10.1136/bmj.l4898
Parums DV (2021) Editorial: review articles, systematic reviews, meta-analysis, and the updated preferred reporting items for systematic reviews and meta-analyses (PRISMA) 2020 Guidelines. Med Sci Monit 27:e934475–1. https://doi.org/10.12659/MSM.934475
Karim A, Muhammad T, Shah I, Khan J, Qaisar R (2022) A multistrain probiotic reduces sarcopenia by modulating Wnt signaling biomarkers in patients with chronic heart failure. J Cardiol 80(5):449–455. https://doi.org/10.1016/j.jjcc.2022.06.006
Pourrajab B, Naderi N, Janani L, Hajahmadi M, Mofid V, Dehnad A, Sohouli MH, Hosseini S, Shidfar F (2022) The impact of probiotic yogurt versus ordinary yogurt on serum sTWEAK, sCD163, ADMA, LCAT and BUN in patients with chronic heart failure: a randomized, triple-blind, controlled trial. J Sci Food Agric 102(13):6024–6035
Awoyemi A, Mayerhofer C, Felix AS, Hov JR, Moscavitch SD, Lappegård KT, Hovland A, Halvorsen S, Halvorsen B, Gregersen I, Svardal A (2021) Rifaximin or Saccharomyces boulardii in heart failure with reduced ejection fraction: results from the randomized GutHeart trial. EBioMedicine 70:103511. https://doi.org/10.1016/j.ebiom.2021.103511
Moludi J, Saiedi S, Ebrahimi B, Alizadeh M, Khajebishak Y, Ghadimi SS (2021) Probiotics supplementation on cardiac remodeling following myocardial infarction: a single-center double-blind clinical study. J Cardiovasc Transl Res 14:299–307. https://doi.org/10.1007/s12265-020-10052-1
Pourrajab B, Naderi N, Janani L, Mofid V, Hajahmadi M, Dehnad A et al (2020) Comparison of probiotic yogurt and ordinary yogurt consumption on serum Pentraxin3, NT-proBNP, oxLDL, and ApoB100 in patients with chronic heart failure: A randomized, triple-blind, controlled trial. Food Funct 11(11):10000–10010
Costanza AC, Moscavitch SD, Faria Neto HCC, Mesquita ET (2015) Probiotic therapy with Saccharomyces boulardii for heart failure patients: a randomized, double-blind, placebo-controlled pilot trial. Int J Cardiol 179:348–350. https://doi.org/10.1016/j.ijcard.2014.11.034
van den Munckhof ICL, Kurilshikov A, Ter Horst R, Riksen NP, Joosten LAB, Zhernakova A et al (2018) Role of gut microbiota in chronic low-grade inflammation as potential driver for atherosclerotic cardiovascular disease: a systematic review of human studies. Obes Rev 19:1719–1734. https://doi.org/10.1111/obr.12750
Kamo T, Akazawa H, Suda W, Saga-Kamo A, Shimizu Y, Yagi H et al (2017) Dysbiosis and compositional alterations with aging in the gut microbiota of patients with heart failure. PLoS One 12:e0174099. https://doi.org/10.1371/journal.pone.0174099
Anderson KM, Ferranti EP, Alagha EC, Mykityshyn E, French CE, Reilly CM (2022) The heart and gut relationship: a systematic review of the evaluation of the microbiome and trimethylamine-N-oxide (TMAO) in heart failure. Heart Fail Rev 27:2223–2249. https://doi.org/10.1007/s10741-022-10254-6
Lv S, Wang Y, Zhang W, Shang H (2022) Trimethylamine oxide: a potential target for heart failure therapy. Heart 108:917–922. https://doi.org/10.1136/heartjnl-2021-320054
Li Z, Wu Z, Yan J, Liu H, Liu Q, Deng Y et al (2019) Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab Investig 99:346–357. https://doi.org/10.1038/s41374-018-0091-y
Boutagy NE, Neilson AP, Osterberg KL, Smithson AT, Englund TR, Davy BM et al (2015) Probiotic supplementation and trimethylamine-N-oxide production following a high-fat diet. Obesity 23:2357–2363. https://doi.org/10.1002/oby.21212
Lu X, Liu J, Zhou B, Wang S, Liu Z, Mei F et al (2022) Microbial metabolites and heart failure: friends or enemies? Front Microbiol 13:956516. https://doi.org/10.3389/fmicb.2022.956516
Permatasari HK, Nurkolis F, Gunawan W Ben, Yusuf VM, Yusuf M, Kusuma RJ et al (2022) Modulation of gut microbiota and markers of metabolic syndrome in mice on cholesterol and fat enriched diet by butterfly pea flower kombucha. Curr Res Food Sci 5:1251–65. https://doi.org/10.1016/j.crfs.2022.08.005
Le Barz M, Anhê FF, Varin TV, Desjardins Y, Levy E, Roy D et al (2015) Probiotics as complementary treatment for metabolic disorders. Diabetes Metab J 39:291–303. https://doi.org/10.4093/dmj.2015.39.4.291
Kelly P, Besa E, Zyambo K, Louis-Auguste J, Lees J, Banda T et al (2016) Endomicroscopic and transcriptomic analysis of impaired barrier function and malabsorption in environmental enteropathy. PLoS Negl Trop Dis 10. https://doi.org/10.1371/journal.pntd.0004600
Kulikov EE, Majewska J, Prokhorov NS, Golomidova AK, Tatarskiy EV, Letarov AV (2017) Effect of O-acetylation of O antigen of Escherichia coli lipopolysaccharide on the nonspecific barrier function of the outer membrane. Microbiol (Russian Fed) 86:310–6. https://doi.org/10.1134/S0026261717030080
Camilleri M, Lyle BJ, Madsen KL, Sonnenburg J, Verbeke K, Wu GD (2019) Role for diet in normal gut barrier function: developing guidance within the framework of food-labeling regulations. Am J Physiol - Gastrointest Liver Physiol 317:G17-39. https://doi.org/10.1152/ajpgi.00063.2019
Chen X-F, Chen X, Tang X (2020) Short-chain fatty acid, acylation and cardiovascular diseases. Clin Sci (London, Engl 1979) 134:657–76. https://doi.org/10.1042/CS20200128
Yukino-Iwashita M, Nagatomo Y, Kawai A, Taruoka A, Yumita Y, Kagami K et al (2022) Short-chain fatty acids in gut-heart axis: their role in the pathology of heart failure. J Pers Med 12. https://doi.org/10.3390/jpm12111805
Marques FZ, Nelson E, Chu PY, Horlock D, Fiedler A, Ziemann M et al (2017) High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 135:964–977. https://doi.org/10.1161/CIRCULATIONAHA.116.024545
Connelly KA, Zhang Y, Visram A, Advani A, Batchu SN, Desjardins JF et al (2019) Empagliflozin improves diastolic function in a nondiabetic rodent model of heart failure with preserved ejection fraction. JACC Basic to Transl Sci 4:27–37. https://doi.org/10.1016/j.jacbts.2018.11.010
Maayah ZH, El-Kadi AOS (2016) The role of mid-chain hydroxyeicosatetraenoic acids in the pathogenesis of hypertension and cardiac hypertrophy. Arch Toxicol 90:119–136. https://doi.org/10.1007/s00204-015-1620-8
Acknowledgements
We offer a great thank you to the Chairman of the Indonesian Association of Clinical Nutrition Physicians and Professor Hardinsyah, Ph.D. (as President of Federations of Asian Nutrition Societies), who has reviewed and provided suggestions with motivational support, as well as input on the draft of this critical review article.
Author information
Authors and Affiliations
Contributions
The conceptualization with the design of the review study and formal analysis: N.A.T., M.Y., and F.N.; visualization and methodology: M.Y. and F.N.; data curation: M.Y., F.N., A.M.A., I.M.D.R.P., and F.Z.I.; supervision: N.A.T., H.H., W.B.G., N.M., and F.N.; drafted-edited-revised-writing and critically revised manuscript: N.A.T., M.Y., F.N., A.M.A., I.M.D.R.P., F.Z.I., H.H., R.K., W.B.G., N.M., T.E.T., B.K., A.T., V.F.F.J., N.S., and M.R.; all authors and contributors has approved the final version of the submitted manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Taslim, N.A., Yusuf, M., Ambari, A.M. et al. Anti-Inflammatory, Antioxidant, Metabolic and Gut Microbiota Modulation Activities of Probiotic in Cardiac Remodeling Condition: Evidence from Systematic Study and Meta-Analysis of Randomized Controlled Trials. Probiotics & Antimicro. Prot. 15, 1049–1061 (2023). https://doi.org/10.1007/s12602-023-10105-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-023-10105-2