Abstract
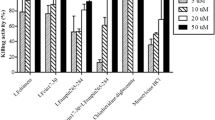
This study evaluated the cytocompatibility and antimicrobial/antibiofilm effects of epigallocatechin-3-gallate (EGCG) associated with peptide LL-37 and its analogue KR-12-a5 against oral pathogens. The effect of the compounds on metabolism of fibroblasts was evaluated by methyltetrazolium assays. Antimicrobial activity of the compounds was evaluated on Streptococcus mutans, Enterococcus faecalis, Actinomyces israelii, and Fusobacterium nucleatum under planktonic conditions, on single- and dual-species biofilms and E. faecalis biofilms in dentinal tubules and analyzed by bacterial counts and confocal microscopy. Data were statistically analyzed considering p < 0.05. EGCG and peptide combinations were not toxic to fibroblasts. KR-12-a5 showed synergistic or addictive effects with EGCG and LL-37 against all bacteria tested. However, EGCG associated with KR-12-a5 demonstrated the highest bactericidal activity on all bacteria tested, at lower concentrations. In single-species biofilms, EGCG + KR-12-a5 eliminated S. mutans and A. israelii and reduced E. faecalis and F. nucleatum counts around 5 log CFU/mL. EGCG + KR-12-a5 reduced E. faecalis (-3.93 log CFU/mL) and eliminated S. mutans in dual-species biofilms. No growth of E. faecalis and significant reduction in A. israelii (−6.24 log CFU/mL) and F. nucleatum (−4.62 log CFU/mL) counts were detected in dual-species biofilms. The combination of EGCG and KR-12-a5 led to 88% of E. faecalis dead cells inside dentin tubules. The association of EGCG and KR-12-a5 was cytocompatible and promoted synergistic effect against biofilms of bacteria associated with endodontic infections.





Similar content being viewed by others
Data Availability
The data that support the findings are part of KSC’s thesis and are available in UNESP Institutional Repository (https://repositorio.unesp.br/).
References
Palombo EA. (2011). Traditional medicinal plant extracts and natural products with activity against oral bacteria: potential application in the prevention and treatment of oral diseases. Evid Based Complement Alternat Med. 2011:680354. https://doi.org/10.1093/ecam/nep067
Bedran TBL, Spolidorio DP, Grenier D (2015) Green tea polyphenol epigallocatechin-3- gallate and cranberry proanthocyanidins act in synergywith cathelicidin (LL-37) to reduce the LPS-induced inflammatory response in a three-dimensional co-culture model of gingival epithelial cells and fibroblasts. Arch Oral Biol 60:845–853. https://doi.org/10.1016/j.archoralbio.2015.02.021
Palaska I, Papathanasiou E, Theoharides TC (2013) Use of polyphenols in periodontal inflammation. Eur J Pharmacol 15(720):77–83. https://doi.org/10.1016/j.ejphar.2013.10.047
Harborne JB, Williams CA (2000) Advances in flavonoid research since 1992. Phytochemistry 55:481–504. Review. https://doi.org/10.1016/s0031-9422(00)00235-1
Nkhili E, Tomao V, El Hajji H, El Boustani ES, Chemat F, Dangles O (2009) Microwave-assisted water extraction of green tea polyphenols. Phytochem Anal 20:408–415. https://doi.org/10.1002/pca.1141
Saito ST, Gosmann G, Pungartnik C, Brendel M (2009) Green tea extract-patents and diversity of uses. Recent Pat Food Nutr Agric 1:203–215. https://doi.org/10.2174/2212798410901030203
Chacko S, Thambi PT, Kuttan R, Nishigaki I (2010) Beneficial effects of green tea: a literature review. Chin Med 5:13. https://doi.org/10.1186/1749-8546-5-13
Legeay S, Rodier M, Fillon L, Faure S, Clere N (2015) Epigallocatechin Gallate: a review of its beneficial properties to prevent metabolic syndrome. Nutrients 7:5443–5468. https://doi.org/10.3390/nu7075230
Song X, Du J, Zhao W, Guo Z (2017) Epigallocatechin-3-gallate (EGCG): mechanisms and the combined applications. Comb Chem High Throughput Screen 17. https://doi.org/10.2174/1386207321666171218115850
Lee P, Tan KS (2015) Effects of Epigallocatechin gallate against Enterococcus faecalis biofilm and virulence. Arch Oral Biol 60:393–399. https://doi.org/10.1016/j.archoralbio.2014.11.014
Xu X, Zhou XD, Wu CD (2012) Tea catechin epigallocatechin gallate inhibits Streptococcus mutans biofilm formation by suppressing gtf genes. Arch Oral Biol 57:678–683. https://doi.org/10.1016/j.archoralbio.2011.10.021
Hölzl MA, Hofer J, Steinberge P, Pfistershammer K, Zlabinger GJ (2008) Host anticrobial proteins as endogenous immunomodulators. Immunol Lett 119:4–11. https://doi.org/10.1016/j.imlet.2008.05.003
Gorr SU, Aldolhosseini M (2011) Antimicrobial peptides and periodontal disease. J Clin Periodontal 38(Suppl 11):126–141. https://doi.org/10.1111/j.1600-051X.2010.01664.x
Dürr UH, Sudheendra US, Ramamoorthy A (2006) LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta 758:1408–1425. https://doi.org/10.1016/j.bbamem.2006.03.030
Zhang Z, Cherryholmes G, Shively JE (2008) Neutrophil secondary necrosis is induced by LL-37 derived from cathelicidin. J Leukoc Biol 84:780–788. https://doi.org/10.1189/jlb.0208086
Mccormick TS (2000) Weinberg A (2010) Epithelial cell-derived antimicrobial peptides are multifunctional agents that bridge innate and adaptive immunity. Periodontol 54:195–206. https://doi.org/10.1111/j.1600-0757.2010.00373.x
Ouhara K, Komatsuzawa H, Yamada S, Shiba H, Fujiwara T, Ohara M, Sayama K, Hashimoto K, Kurihara H, Sugai M (2005) Susceptibilities of periodontopathogenic and cariogenic bacteria to antibacterial peptides, {beta}-defensins and LL37, produced by human epithelial cells. J Antimicrob Chemother 55:888–896. https://doi.org/10.1093/jac/dki103
Caiaffa KS, Massunari L, Danelon M, Abuna GF, Bedran TBL, Santos-Filho NA, Spolidorio DMP, Vizoto NL, Cilli EM, Duque C (2017) KR-12-a5 is a non-cytotoxic agent with potent antimicrobial effects against oral pathogens. Biofouling 33:807–818. https://doi.org/10.1080/08927014.2017.1370087
Harder J, Bartels J, Christophers E, Schroder JM (2001) Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem 23(276):5707–5713. https://doi.org/10.1074/jbc.M008557200
Wong JH, Ng TB, Legowska A, Rolka K, Hui M, Cho CH (2011) Antifungal action of human cathelicidin fragment (LL13-37) on Candida albicans. Peptides 32:1996–2002. https://doi.org/10.1016/j.peptides.2011.08.018
Suphasiriroj W, Mikami M, Shimomura H, Sato S (2013) Specificity of antimicrobial peptide LL-37 to neutralize periodontopathogenic lipopolysaccharide activity in human oral fibroblasts. J Periodontol 84:256–264. https://doi.org/10.1902/jop.2012.110652
Wang G (2008) Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J Biol Chem 21(283):32637–32643. https://doi.org/10.1074/jbc.M805533200
Jacob B, Park IS, Bang JK, Shin SY (2013) Short KR-12 analogs designed from human cathelicidin LL37 possessing both antimicrobial and antiendotoxicactivities without mammalian cell toxicity. J Pept Sci 19:700–707. https://doi.org/10.1002/psc.2552
Harasstani OA, Moin S, Tham CL, Liew CY, Ismail N, Rajajendram R, Harith HH, Zakaria ZA, Mohamad AS, Sulaiman MR, Israf DA (2010) Flavonoid combinations cause synergistic inhibition of proinflammatory mediator secretion from lipopolysaccharide-induced RAW 264.7 cells. Inflamm Res 59:711–721. https://doi.org/10.1007/s00011-010-0182-8
Feldman M, Grenier D (2012) Cranberry proanthocyanidins act in synergy with licochalcone A to reduce Porphyromonas gingivalis growth and virulence properties, and to suppress cytokine secretion by macrophages. J Appl Microbiol 113:438–447. https://doi.org/10.1111/j.1365-2672.2012.05329.x
Guo YJ, Zhang B, Feng XS, Ren HX, Xu JR (2016) Human cathelicidin LL-37 enhance the antibiofilm effect of EGCG on Streptococcus mutans. BMC Oral Health 22(16):101. https://doi.org/10.1186/s12903-016-0292-y
Ji S, Hyun J, Park E, Lee BL, Kim KK, Choi Y (2007) Susceptibility of various oral bacteria to antimicrobial peptides and to phagocytosis by neutrophils. J Periodontal Res 42:410–419. https://doi.org/10.1111/j.1600-0765.2006.00962.x
CLSI – Clinical and Laboratory Standard Institute (2012) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. 9thed. Wayne, PA, CLSI document, M7-A9
Mor A, Hani K, Nicolas PJ (1994) The vertebrate peptide antibiotics dermaseptins have overlapping structural features but target specific microorganisms. J Biol Chem 269:31635–31641
Tong Z, Zhou L, Jiang W, Kuang R, Li J, Tao R, Ni L (2011) An in vitro synergetic evaluation of the use of nisin and sodium fluoride or chlorhexidine against Streptococcus mutans. Peptides 32:2021–2026. https://doi.org/10.1016/j.peptides.2011.09.002
Kim EY, Rajasekaran G, Shin SY (2017) LL-37-derived short antimicrobial peptide KR-12-a5 and its d-amino acid substituted analogs with cell selectivity, anti-biofilm activity, synergistic effect with conventional antibiotics, and anti-inflammatory activity. Eur J Med Chem 18(136):428–441. https://doi.org/10.1016/j.ejmech.2017.05.028
Soares DG, Marcomini N, Basso FG, Pansani TN, Hebling J, de Souza Costa CA (2014) Indirect cytocompatibility of a low-concentration hydrogen peroxide bleaching gel to odontoblast-like cells. Int Endod J 49:26–36. https://doi.org/10.1111/iej.12426
Massunari L, Novais RZ, Oliveira MT, Valentim D, Dezan Junior E, Duque C (2017) Antimicrobial activity and biocompatibility of the Psidium cattleianum extracts for endodontic purposes. Braz Dent J 28:372–379. https://doi.org/10.1590/0103-6440201601409
Gao Y, Jiang X, Lin D, Chen Y, Tong Z (2016) The starvation resistance and biofilm formation of Enterococcus faecalis in Coexistence with Candida albicans, Streptococcus gordonii, Actinomyces viscosus, or Lactobacillus acidophilus. J Endod 42:1233–1238. https://doi.org/10.1016/j.joen.2016.05.002
Ma J, Wang Z, Shen Y, Haapasalo M (2011) A new noninvasive model to study the effectiveness of dentin disinfection by using confocal laser scanning microscopy. J Endod 37:1380–1385. https://doi.org/10.1016/j.joen.2011.06.018
Scott MG, Vreugdenhil AC, Buurman WA, Hancock RE, Gold MR (2000) Cutting edge: cationic antimicrobial peptides block the binding of lipopolysaccharide (LPS) to LPS binding protein. J Immunol 164(2):549–553. https://doi.org/10.4049/jimmunol.164.2.549
Bedran TBL, Mayer MP, Spolidorio DP, Grenier D (2014) Synergistic anti-inflammatory activity of the antimicrobial peptides human β-defensin-3 (hBD-3) and cathelicidin (LL-37) in a three-dimensional co-culture model of gingival epithelial cells and fibroblasts. PLoS One 4(9):e106766. https://doi.org/10.1371/journal.pone.0106766
da Silva BR, Conrado AJS, Pereira AL, Evaristo FFV, Arruda FVS, Vasconcelos MA, Lorenzón EN, Cilli EM, Teixeira EH (2017) Antibacterial activity of a novel antimicrobial peptide [W7]KR12-KAEK derived from KR-12 against Streptococcus mutans planktonic cells and biofilms. Biofouling 33:835–846. https://doi.org/10.1080/08927014.2017.1374378
Budzik JM, Schneewind O (2006) Pili prove pertinent to enterococcal endocarditis. J Clin Invest 116:2582–2584. https://doi.org/10.1172/JCI30088
Van den Berghe E, De Winter T, De Vuyst L (2006) Enterocin A production by Enterococcus faecium FAIR-E 406 is characterised by a temperature- and pH-dependent switch-off mechanism when growth is limited due to nutrient depletion. Int J Food Microbiol 107:159–170. https://doi.org/10.1016/j.ijfoodmicro.2005.08.027
Nakajo K, Iwami Y, Komori R, Ishikawa S, Ueno T, Suzuki Y, Takahashi N (2005) The resistance to acidic and alkaline environments of endodontic pathogen Enterococcus faecalis. Int Congr Ser 1284:191–192
Yap B, Zilm PS, Briggs N, Rogers AH, Cathro PC (2014) The effect of sodium hypochlorite on Enterococcus faecalis when grown on dentine as a single- and multi-species biofilm. Aust Endod J 40:101–110. https://doi.org/10.1111/aej.12073
Lee CC, Sun Y, Qian S, Huang HW (2011) Transmembrane pores formed by human antimicrobial peptide LL-37. Biophys J 6(100):1688–1696. https://doi.org/10.1016/j.bpj.2011.02.018
Nguyen LT, Haney EF, Vogel HJ (2011) The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol 29:464–472. https://doi.org/10.1111/j.1399-302X.2006.00289.x
Yoda Y, Hu ZQ, Zhao WH, Shimamura T (2004) Different susceptibilities of Staphylococcus and Gram-negative rods to epigallocatechin gallate. J Infect Chemother 10:55–58. https://doi.org/10.1007/s10156-003-0284-0
Cui Y, Oh YJ, Lim J, Youn M, Lee I, Pak HK, Park W, Jo W, Park S (2012) AFM study of the differential inhibitory effects of the green tea polyphenol (-)-epigallocatechin-3-gallate (EGCG) against Gram-positive and Gram-negative bacteria. Food Microbiol 29:80–87. https://doi.org/10.1016/j.fm.2011.08.019
Funding
This study was supported by CNPq (# 140467/2018–1), FAPESP (#2013/07600–3), and CAPES (financial code #01 and CAPES-PROCAD # 88881.068437/2014–01).
Author information
Authors and Affiliations
Contributions
C.D. and K.S.C. designed all preliminary experiments. C.D., K.S.C., and G.F.A. revised the microbiology experiment design and organized and analyzed the data. K.S.C., V.R.S., G.F.A., and N.A.S. performed all the study experiments and interpreted the project results. C.D. and K.S.C. wrote the initial draft of the manuscript. E.M.C., V.T.S., and L.T.A.C. revised critically the manuscript, and C.D. and K.S.C. performed the final revision of the manuscript prior to its submission.
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with human participants performed by any of the authors. This study was approved by the Animal Committee of Araçatuba Dental School, UNESP, Brazil (FOA: 01194–2017).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Caiaffa, K.S., dos Santos, V.R., Abuna, G.F. et al. Cytocompatibility and Synergy of EGCG and Cationic Peptides Against Bacteria Related to Endodontic Infections, in Planktonic and Biofilm Conditions. Probiotics & Antimicro. Prot. 13, 1808–1819 (2021). https://doi.org/10.1007/s12602-021-09830-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-021-09830-3