Abstract
Objectives
We evaluated several flavonoid combinations for synergy in the inhibition of proinflammatory mediator synthesis in the RAW 264.7 cellular model of inflammation.
Methods
The inhibitory effect of chrysin, kaempferol, morin, silibinin, quercetin, diosmin and hesperidin upon nitric oxide (NO), prostaglandin E2 (PGE2) and tumour necrosis factor-α (TNF-α) secretion from the LPS-induced RAW 264.7 monocytic macrophage was assessed and IC50 values obtained. Flavonoids that showed reasonable inhibitory effects in at least two out of the three assays were combined in a series of fixed IC50 ratios and reassessed for inhibition of NO, PGE2 and TNF-α. Dose–response curves were generated and interactions were analysed using isobolographic analysis.
Results
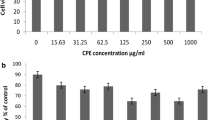
The experiments showed that only chrysin, kaempferol, morin, and silibinin were potent enough to produce dose–response effects upon at least two out of the three mediators assayed. Combinations of these four flavonoids showed that several combinations afforded highly significant synergistic effects.
Conclusions
Some flavonoids are synergistic in their anti-inflammatory effects when combined. In particular chrysin and kaempferol significantly synergised in their inhibitory effect upon NO, PGE2 and TNF-α secretion. These findings open further avenues of research into combinatorial therapeutics of inflammatory-related diseases and the pharmacology of flavonoid synergy.







Similar content being viewed by others
Abbreviations
- NO:
-
Nitric oxide
- PGE2 :
-
Prostaglandin E2
- TNF-α:
-
Tumour necrosis factor-α
- LPS:
-
Lipopolysaccharide
- IC50 :
-
Inhibitory concentration 50
- DMSO:
-
Dimethyl sulfoxide
- DMEM:
-
Dulbecco’s modified eagle media
- MTT:
-
3-[4, 5-Dimethyl-2-thiazolyl]-2,5-diphenyl tetrazolium bromide
- FBS:
-
Foetal bovine serum
- L-NAME:
-
N-nitro-l-arginine methyl ester
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- MIC:
-
Minimal inhibitory concentration
- EGCG:
-
Epigallocatechin gallate
- NSAID:
-
Non-steroidal anti-inflammatory drug
- MAPK:
-
Mitogen-activated protein kinase
- IRF-1:
-
Interferon regulatory factor-1
- ERK:
-
Extracellular-regulated kinase
- JNK:
-
c-Jun N-terminal kinase
- LDL:
-
Low density lipoprotein
References
Kim HP, Son KH, Chang HW, Kang SS. Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci. 2004;96:229–45.
Williamson EM. Synergy and other interactions in phytomedicines. Phytomed. 2001;8:401–9.
Ting-Chao Chou. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–81.
Scott EN, Gescher AJ, Steward WP, Brown K. Development of dietary phytochemical chemopreventive agents: biomarkers and choice of dose for early clinical trials. Cancer Prev Res (Phila Pa). 2009;2:525–30.
Duarte J, Perez-vizcaino F, Jimenez J, Tamargo J, Zarzuelo A. Flavonoids and cardiovascular diseases. Stud Nat Prod Chem. 2001;25:565–605.
Galvez J, De Sanchez Medina F, Jimenez J, Zarzuelo A. Effects of flavonoids on gastrointestinal disorders. Stud Nat Prod Chem. 2001;25:607–49.
Schroeter H, Boyd C, Spencer JPE, Williams RJ, Cadenas E, Rice-Evans C. MAPK signaling in neurodegeneration: influences of flavonoids and of nitric oxide. Neurobiol Aging. 2002;23:861–80.
Gohel MS, Davies AH. Pharmacological agents in the treatment of venous disease: an update of the available evidence. Curr Vasc Pharmacol. 2009;7:303–8.
Sato Y, Shibata H, Arai T, Yamamoto A, Okimura Y, Arakaki N, et al. Variation in synergistic activity by flavone and its related compounds on the increased susceptibility of various strains of methicillin-resistant Staphylococcus aureus to β-lactam antibiotics. Int J Antimicro Ag. 2004;24:28–35.
Scambia G, De Vincenzo R, Ranelletti FO, Benedetti Panici P, Ferrandina G, D’Agostino G, et al. Antiproliferative effect of silybin on gynaecological malignancies: synergism with cisplatin and doxorubicin. Eur J Canc. 1996;32A:877–82.
Milde J, Elstner EF, Graßmann J. Synergistic inhibition of low-density lipoprotein oxidation by rutin, γ-terpinene, and ascorbic acid. Phytomed. 2004;11:105–13.
Wang Q, Han Y, Xue H. Ligands of the GABAA receptor benzodiazepine binding site. CNS Drug Rev. 1999;5:124–44.
Medina JH, Viola H, Wolfman C, Marder M, Wasowski C, Calvo D, et al. Overview. Flavonoids: a new family of benzodiazepine receptor ligands. Neurochem Res. 1997;22:419–25.
Marder M, Paladini AC. GABAA-receptor ligands of flavonoid structure. Curr Top Med Chem. 2002;2:853–67.
Fernandez SP, Wasowski C, Paladini AC, Marder M. Synergistic interaction between hesperidin, a natural flavonoid, and diazepam. Eur J Pharmacol. 2005;512:189–98.
Murakami A, Takahashi D, Hagihara K, Koshimizu K, Ohigashi H. Combinatorial effects of nonsteroidal anti-inflammatory drugs and food constituents on production of prostaglandin E2 and tumour necrosis factor–α in RAW 264.7 murine macrophages. Biosci Biotechnol Biochem. 2003;67:1056–62.
Tallarida RJ, Porreca F, Cowan A. Statistical analysis of drug-drug and site-site interactions with isobolograms. Life Sci. 1989;45:947–61.
Pinardi G, Sierralta F, Miranda HF. Interaction between the antinociceptive effect of ketoprofen and adrenergic modulatory systems. Inflamm. 2001;25:233–9.
Tallarida RJ. Drug synergism: its detection and applications. J Pharmacol Exp Ther. 2001;298:865–72.
Dehmlow C, Murawski N, De Groot H. Scavenging of reactive oxygen species and inhibition of arachidonic acid metabolism by silibinin in human cells. Life Sci. 1996;58:l591–1600.
Wadsworth TL, McDonald TL, Koop DR. Effects of Ginkgo biloba extract (EGb 761) and quercetin on lipopolysaccharide-induced signaling pathways involved in the release of tumor necrosis factor-α. Biochem Pharmacol. 2001;62:963–74.
Cho SY, Park SJ, Kwon MJ, Jeong TS, Bok SH, Choi WY, et al. Quercetin suppresses proinflammatory cytokines production through MAP kinases and NF-κB pathway in lipopolysaccharide-stimulated macrophage. Mol Cell Biochem. 2003;243:153–60.
Takano-Ishikawa Y, Goto M, Yamaki K. Structure–activity relations of inhibitory effects of various flavonoids on lipopolysaccharide-induced prostaglandin E2 production in rat peritoneal macrophages: Comparison between subclasses of flavonoids. Phytomed. 2006;13:310–7.
Hamalainen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E. Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm 2007;45673–45683.
Park HH, Lee S, Son HY, Park SB, Kim MS, Choi EJ, et al. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch Pharm Res. 2008;31:1303–11.
Crespo I, García-Mediavilla MV, Gutiérrez B, Sánchez-Campos S, Tuñón MJ, González-Gallego J. A comparison of the effects of kaempferol and quercetin on cytokine-induced pro-inflammatory status of cultured human endothelial cells. Br J Nutr. 2008;100:968–76.
Hecker M, Priess C, Klemm P, Brusse R. Inhibition by antioxidants of nitric oxide synthase expression in murine macrophages; role of nuclear factor kappa B and interferon regulatory factor 1. Br J Pharmacol. 1996;118:21178–84.
Chen CC, Chow MP, Huang WC, Lin YC, Chang YJ. Flavonoids inhibit tumor necrosis factor-α-induced up-regulation of intercellular adhesion molecule-1 (ICAM-1) in respiratory epithelial cells through activator protein-1 and nuclear factor-κB: structure-activity relationships. Mol Pharmacol. 2004;66:683–93.
Bowie A, O’Neill LAJ. Oxidative stress and nuclear factor-κB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol. 2000;59:13–23.
Acknowledgments
We thank Zulkhairi Zainol, Abdul Rahman Hassan and Nora Asyikin Mohd Salim for technical assistance. This investigation was financially supported by Science Fund (06-01-04-SF0973), Ministry of Science, Technology and Innovation, Malaysia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: J. Skotnicki.
Rights and permissions
About this article
Cite this article
Harasstani, O.A., Moin, S., Tham, C.L. et al. Flavonoid combinations cause synergistic inhibition of proinflammatory mediator secretion from lipopolysaccharide-induced RAW 264.7 cells. Inflamm. Res. 59, 711–721 (2010). https://doi.org/10.1007/s00011-010-0182-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-010-0182-8