Abstract
Button mushroom (Agaricus bisporus) is the predominant mushroom species cultivated around the world. In the button mushroom cultivation, casing soil is one of the main substrate inducing emergence of mushrooms but presence of Trichoderma aggressivum f. aggressivum (causal agent of green mould disease) in casing soil causes devastating yield losses. However, little is known about management of the green mould in button mushroom cultivation. The aim of this study was to examine efficacy of several disinfectants and heat treatments against T. aggressivum f. aggressivum in casing soil and mushroom yield. In this respect, by considering yield (total amount of sporophores) values, in vivo experiments were separately set up according to randomized block design with three replications. As a result, compared to controls, disinfectants [hydrogen peroxide (H202), formaldehyde (CH2O), sodium hypochlorite (NaOCl) and hypochlorous acid (HOCl)] and heat treatments (60, 90 and 120 °C) significantly (P˂0.01) increased mushroom yields up to 18.74 and 24.06% in the treated plots, respectively. Biological efficiency values ranged from 87.16 to 105.72% in the disinfectant treatments, while they varied from 93.15 to 95.68% in the heat treatments. However, applications of the disinfectants at high doses had negative influence on growth of A. bisporus. The overall results suggest that the tested disinfectants and heat treatments may significantly increase button mushroom yield by suppressing development of T. aggressivum f. aggressivum in casing soil. The present study not only reveals management practices that can be used against the green mould in the in vivo but also presents new knowledge for mushroom industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trichoderma species, the causal agents of the green mould disease, induce epidemics in button mushroom (Agaricus bisporus) cultivation around the world. In the epidemics, a wide range of Trichoderma (e.g. T. harzianum, T. atroviride, T. koningii, T. virens, T. mienum and T. aggressivum) were reported associated with the green mould (Kim et al., 2012; Kosanović et al., 2013). However, Trichoderma aggressivum f. europaeum and T. aggressivum f. aggressivum are considered as aggressive biotypes of the green mould in Europe and North America, respectively (Hatvani et al., 2007). To date, Trichoderma aggressivum f. aggressivum has been known to occur in North America, but it was also reported in Hungary and Türkiye (Hatvani et al., 2017; Aydoğdu et al., 2020), implying that apart from North America, T. aggressivum f. aggressivum is spreading and posing threat to other button mushroom growing countries.
Trichoderma species including T. aggressivum can be found in different soils in various agroecological areas (e.g. gardens, orchards, fields, forests) and in casing soil used in button mushroom cultivation (Błaszczyk et al., 2011; Mirkhani & Alaei, 2015). Presence of the green mould in casing soil inhibits fruiting body (sporophore) formation of A. bisporus. Thus, yield losses occur in button mushroom cultivation (Lee et al., 2014). Although the green mould disease particularly its aggressive biotypes (T. aggressivum) cause epidemics and devastating yield losses in button mushroom cultivation. Little is known about management of the disease.
In the management of fungal diseases (green mould, dry bubble, wet bubble and cobweb) of button mushroom, several fungicides prochloraz-Mn and metrafenone in Europe and thiabendazol, tiophanate-methyl and chlorothalonil in the United States and Canada have been officially recommended (Hatvani et al., 2012; Šantrić et al., 2018). These fungicides are applied to casing soil. However, fungicide resistance of the causal agents in button mushroom cultivation has been frequently reported (Berendsen et al., 2010; Medvediev et al., 2019). Apart from this, toxic effects of fungicides on button mushroom (Kosanović et al., 2015) should not be overlooked as well. As regards other alternative management practices tested, Potočnik et al. (2014) reported efficacy of peracetic acid in the management of cobweb disease of button mushroom. In addition, essential oils were tested against several fungi (Trichoderma harzianum, Trichoderma agressivum f. europaeum, Verticillium fungicola, Cladobotryum, Mycogone, Lecanicillium and Trichoderma) in the in vitro studies (Soković & van Griensven, 2006; Górski et al., 2010; Đurović-Pejčev et al., 2014; Geösel et al., 2014; Mehrparvar et al., 2016; Santos et al., 2017; Diánez et al., 2018; Gea et al., 2019). Results of these experiments seemed to be efficient but negative influence of the essential oils on mycelial growth of A. bisporus, cost and application difficulties in the in vivo created difficulties in their use in button mushroom cultivation (Geösel et al., 2014; Santos et al., 2017; Gea et al., 2019). Moreover, Yang et al. (2019) emphasized that A. bisporus absorbed volatile compounds during storage, indicating that button mushroom may absorb essential oils used in the management of the fungal diseases during button mushroom cultivation. Consequently, this may create marketing problems due to odour and taste of button mushroom. Thus, other alternative management practices that are applicable in the in vivo, cost-effective, non-toxic to A. bisporus and environmentally friendly should be considered for the management of the green mould. In this regard, disinfectants [hydrogen peroxide (H202), formaldehyde (CH2O), sodium hypochlorite (NaOCl) and hypochlorous acid (HOCl)] and heat treatments (60, 90 and 120 °C) were selected in the study. The objective of this study was to investigate efficacy of these disinfectants and the heat treatments against T. aggressivum f. aggressivum in casing soil and mushroom yield.
Materials and methods
Green mould inoculum
Green mould isolate 467 [Accession number: MN177932 in Genbank (http://www.ncbi.nlm.nih.gov)] of Trichoderma aggressivum f. aggressivum was used as inoculum source in the experiments. This isolate was obtained from the culture collection of mycology laboratory of Batı Akdeniz Agricultural Research Institute, Antalya province, Türkiye.
Agaricus b isporus (button mushroom) strain, compost and casing soil
Commercial white (192,915 AG, Soc, France) strain of A. bisporus, compost (trade mark: Ersanlar) and casing soil (trade mark: Çivril) were used for button mushroom cultivation in the experiments. These materials were obtained from SMS Ersanlar mushroom company in Korkuteli county, Antalya province.
In addition, to determine constituents of the mushroom compost and casing soil, one sample (2 kg) for the each material was taken and analyzed at the laboratory of soil and plant nutrition department of Batı Akdeniz Agricultural Research Institute (Table 1).
Disinfectants and material used for heat treatments
Four disinfectants (hydrogen peroxide, formaldehyde, sodium hypochlorite and hypochlorous acid) were used in disinfectant experiments (Table 2).
An autoclave (Alp-CL-32 L, Alp Co. Ltd., Japan) was used for heat application experiments. In this respect, three heat treatments (at 60, 90 and 120 °C for 30 min) were separately applied to casing soil using steam pressure of the autoclave.
Preparation of the green mould inoculum
Green mould isolate 467 (T. aggressivum f. aggressivum) on potato dextrose agar (PDA) was incubated at 25 °C for 6 days in an incubator (Thermo Scientific, Thermo Electron LED GmbH, Germany). Ten millilitre distilled sterile water was put per Petri plate (9 cm in diameter) and developing colony surfaces of T. aggressivum f. aggressivum on PDA were gently scraped through a sterile brush. Spore suspension was filtered through sterile two-layer cheesecloth and adjusted to 1 × 106 conidia/mL using a hemacytometer (Neubauer, Isolab, Germany).
Preparation of conditions for the experiments
Each experiment either disinfectant or heat treatment was separately conducted in a room (4 × 5 m) in the basement of Department of Plant Health of Batı Akdeniz Agricultural Reseach Institute, Antalya province. Air conditioning, ventilation and humidification of the room were provided for button mushroom cultivation. Each experiment was repeated once and set up according to randomized block design with three replications.
Disinfectant treatments
Experimental units were composed of bags (0.2 × 0.35 × 0.2 m) containing 2 kg compost and 650 g casing soil forming a soil layer (3.5–4 cm in thickness) on the top of the compost (Fig. 1).
Initially, 2 kg compost spawned at 2% (w/w) with white strain (192,915 AG, Soc, France) of A. bisporus was added into each bag and incubated at 25 °C for 20 days. Within this period, 2 kg compost in the bag was colonized by A. bisporus. Ensuing this, 650 g casing soil per bag was laid to the top of the 2 kg compost. In this way, a casing soil layer in 3.5 to 4 cm thickness was formed on the compost. Afterwards, 1 mL green mould inoculum (spore suspension; 1 × 106 conidia/mL) per bag was injected into central part of the casing soil using a syringe (Aydoğdu et al., 2021). One day later, three doses of each disinfectant listed below were separately applied to the casing soil in each bag in the treatments. Six mL of each disinfectant was sprayed on surface (0.03m2) of the casing soil using a hand sprayer in each separate treatment.
-
1
-Hypochlorous acid (HOCl): 0.1, 0.5 and 1%.
-
2
-Hydrogen peroxide (H202) : 0.01, 0.1 and 1%.
-
3
-Formaldehyde (CH2O) : 0.1, 0.5 and 1%.
-
4
-Sodium hypochlorite (NaOCl): 1, 5 and 10%.
In the controls, 1 mL spore suspension (1 × 106 conidia/mL) of the isolate T. aggressivum f. aggressivum per bag was injected into the casing soil without disinfectant treatment. Conditions of the room were kept at 22 to 25 °C with 85 to 90% relative humidity for ten days. During this period, sterile distilled water was sprayed on the casing soil at three days intervals to form suitable emergence conditions for A. bisporus. After this ten day-period, temperature of the room was reduced 1 °C per day until 17 °C. Ventilation providing oxygen from out into the room was initiated to reduce carbondioxide in the room. Thus, formation of primordia and then sporophores of A. bisporus were stimulated. After emergence of sporophores, mushrooms reaching marketing size in each bag were picked by hand and weighed. In this way, total yield values for each bag were calculated in the treatments and the controls. Total amount of two flushes of button mushroom were evaluated and mean yield of each treatment was compared with its control.
Heat treatments
As beforementioned in the disinfectant treatments, experimental units were set up for the heat treatments but before adding casing soil to the top of the compost, 650 g casing soil was put into one autoclave bag. Later, 1 mL green mould inoculum (spore suspension; 1 × 106 conidia/mL) was injected into the casing soil in each autoclave bag and exposed at 60 ºC for 30 min in the autoclave. Afterwards, the 650 g casing soil was laid to top of the compost for each bag in the room. In the controls, no heat treatment was applied, 650 g casing soil per bag was laid to the top of the 2 kg compost and 1 mL spore suspension (1 × 106 conidia/mL) of the isolate T. aggressivum f. aggressivum per bag was injected into the casing soil. In the same way, the other two heat treatments (at 90 and 120 ºC 30 min) were set up separately. Later, as mentioned in the disinfectant treatments, the same mushroom growing conditions were maintained and yield values were calculated according to two flushes. Mean yield of each heat treatment was compared with its control plot.
Assessment of biological efficiency of all the treatments
In addition, for further analyzing efficacy of the disinfectants and heat treatments on the mushroom yield, biological efficiency (BE) values were calculated according to the formula below.
BE (%) = Fresh weight of total fruiting body yield ÷ weight of dry spawned substrate mass × 100 (Chrysayi-Tokousbalides et al., 2007).
Statistical analysis
Analysis of variance was performed using SAS 9.1 software program (SAS Institute Inc., Cary, NC, USA). Means of disinfectants (hydrogen peroxide, formaldehyde, sodium hypochlorite and hypochlorous acid), heat treatments (60, 90 and 120 °C), rates of change and biological efficiency values were grouped using Tukey’s multiple range test with the significance of P < 0.01.
Results
Disinfectant treatments
1-Hypochlorous acid (HOCl) treatment
Mean yields of the first, second and third doses of hypochlorous acid treatment were 658.33, 670.33 and 641 g in the treated plots, respectively, whereas they were 570, 589 and 605 g in their control plots. Mean yield differences between the each dose of hypochlorous acid treatments and the control plots were significant (P˂0.01). Compared to the controls, the first and second doses of hypochlorous acid led to the highest yield increase, 15.49 and 13.8%, respectively, but the lowest yield increase (5.95%) was detected in the third dose of hypochlorous acid in the treated plots (Fig. 2).
Comparison of mushroom yield (total amount of sporophores) in each dose of the tested disinfectants [hydrogen peroxide (H202), formaldehyde (CH2O), sodium hypochlorite (NaOCl) and hypochlorous acid (HOCl)] and heat treatments (60, 90 and 120 °C) in the treated and control plots. Values of the bars are means of three replicates and levels not connected by same letter are significantly (P< 0.01) different according to Tukey’s multiple range test
2-Hydrogen peroxide (H2O2) treatment
Compared to the controls, the highest yield increase (14.21%) was found in the second dose of hydrogen peroxide in the treated plots but this yield increase was not different from that of the first dose (P < 0.01) statistically. As for the third dose of hydrogen peroxide, although the third dose created a 5.84% yield increase in the treated plots but mean yield of the treated plots was not different from that of their controls (P < 0.01) statistically. In this context, mean yields of the second, first and third doses of hydrogen peroxide were 605.33, 579.66 and 567.33 g in the treated plots, respectively (Fig. 2).
3-Formaldehyde (CH2O) treatment
In the formaldehyde treatment, compared to the controls, the first and second doses of formaldehyde caused significant (P˂0.01) yield increases up to 18.74 and 12.40% by suppressing T. aggressivum f. aggressivum in the treatments. Mean mushroom yields of these doses of formaldehyde treatments were 591.33 and 576.66 g in the treated plots, respectively, whereas mean yields of their control plots were 498 and 513 g, respectively. Compared to the controls, the third dose of formaldehyde treatment formed a 5.26% yield increase in the treated plots but it was not significant (P˂0.01) statistically (Fig. 2).
4- Sodium hypochlorite (NaOCl) treatment
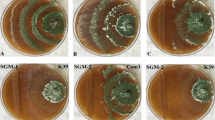
In this treatment, compared to the controls, significant (P < 0.01) differences between the three doses of sodium hypochlorite were established but the first dose led to highest yield increase up to 17.20% in the treated plots. Third dose of sodium hypochlorite created a 1.34% yield increase in the treated plots, but it was not significant (P < 0.01) statistically. Mean yield values of the first, second and third doses of sodium hypochlorite treatments were 610.66, 596.66 and 554.33 g in the treated plots, respectively, while they were 521, 553 and 547 g in their control plots (Fig. 2). One photograph (Fig. 3) from sodium hypochlorite treatment was given as an example for yield comparison of the disinfectant treatments.
Comparison of mushroom yield (total amount of sporophores) in the treated and control plots. A) Yield of the bag (experimental unit) in the treatment (initially inoculated with Trichoderma aggressivum f. aggressivum isolate 467 and then treated with sodium hypochlorite); B) Yield of the bag in the control (inoculated with Trichoderma aggressivum f. aggressivum isolate 467 without sodium hypochlorite application), casing soil layer (orange arrow) and compost (blue arrow)
Heat treatments
Efficacy of heat treatments against T. aggressivum f. aggressivum in casing soil and mushroom yield was investigated by testing three different heat treatments. In this context, compared to the control plots, all the three heat treatments (at 60, 90 and 120 °C for 30 min) led to significant (P˂0.01) yield increases in the treated plots. The highest yield increase (24.06%) was found in the second heat treatment of 90 °C, while the lowest yield increase (16.73%) was detected in the first heat treatment of 60 °C, but the differences between the each heat treatment were not significant statistically. Mean yield values of the heat treatments of 90, 120 and 60 °C were 606.66, 601.33 and 590.66 g, respectively, while mean yields of their control plots were 489, 492 and 506 g in the control plots. The results showed that all the heat treatments tested had suppressive effects on growth of T. aggressivum f. aggressivum in casing soil and promoting mushroom yield as well (Fig. 2).
Overall comparison of all the treatments and their biological efficiency
Compared to the control plots, all the tested experiments induced considerable yield increases in button mushroom (A. bisporus) yield by suppressing growth of the green mould (T. aggressivum f. aggressivum) in casing soil. However, comparing all the experiments, the heat treatments led to higher yield increases than majority of the disinfectant treatments. In the disinfectant treatments, the highest yield increases were detected at the rates of 18.74 and 17.20% in the first doses of the formaldehyde (CH2O) and sodium hypochlorite (NaOCl) treatments, respectively. In general, compared to the other doses of the tested disinfectants, the third doses led to lower yield increases in the treated plots, implying negative effects of increasing doses of the tested disinfectants on A. bisporus growth in casing soil as well (Fig. 2).
Biological efficiency (BE) values of majority of the treatments were significantly higher than those of the controls. For example, BE of the first dose of sodium hypochlorite (NaOCl) in the control was 82.17%, while it was 96.31% in the treated plots. However, there were significant differences among the treatments. In this context, the highest BE values, 105.72, 103.83 and 101.1%, were found in the second, third and first doses of the hypochlorous acid (HOCl) treatments, respectively, but the differences were not significant statistically. The lowest BE values, 93.26, 90.95 and 87,16%, were established in the first, second and third doses of the formaldehyde (CH2O) treatments. Except for the third doses of the sodium hypochlorite (NaOCl), formaldehyde (CH2O) and hydrogen peroxide (H202), all the doses of all the treatments provided over 90% biological efficiency (Fig. 4).
Comparison of biological efficiency (BE) for the tested disinfectants [hydrogen peroxide (H202), formaldehyde (CH2O), sodium hypochlorite (NaOCl) and hypochlorous acid (HOCl)] and heat treatments (60, 90 and 120 °C) in the treated and control plots. Values of the bars are means of three replicates and levels not connected by same letter are significantly (P<0.01) different according to Tukey’s multiple range test
Discussion
Positive effects of hydrogen peroxide (H202) on quality of button mushroom in post-harvest storage were previously reported (Sharaf-Eldin & Geösel, 2016). In the present study, 0.01 and 0.1% concentrations of hydrogen peroxide caused significant (P < 0.01) yield increases up to 14.21% in the treatments, while the 1% concentration induced lower yield increase than the other doses, indicating that increasing dose of hydrogen peroxide may have negatively affected growth of A. bisporus. Likewise, Qiu et al. (2017) reported that hydrogen peroxide at high doses inhibited growth of oyster mushroom strains (e.g. Pleurotus sajor-caju, P. salmoneo-stramineus, P. ostreatus) and caused toxicity, which corroborated the results of the present study. Similarly, in the sodium hypochlorite (NaOCl) experiment, as the dose increased, yield of button mushroom decreased in the treated plots. In this regard, Oh et al. (2000) reported that high doses of sodium hypochlorite inhibited development of oyster mushroom (Pleurotus ostreatus), which is in agreement with the results of the present study.
In the formaldehyde (CH2O) treatment, the similar results aforementioned were also found. Compared to the control, 0.1 and 0.5% concentrations of formaldehyde caused significant (P˂0.01) yield increases in the treatment plots. In this context, Sharma et al. (2007) suggested treatment of casing soil with formaldehyde against soilborne fungus (Lecanicillium fungicola) in button mushroom cultivation, supporting the results of the present study. These findings may imply applicability of formaldehyde against fungi in casing soil in button mushroom cultivation. In the hypochlorous acid (HOCl) experiment, 0.1 and 0.5% concentrations of HOCl created the highest yield increases, whereas the 1% concentration led to significantly lowest yield increase in the treated plots, indicating adverse effects of increasing dose of hypochlorous acid on A. bisporus growth in casing soil. In this respect, Eryılmaz and Palabıyık (2013) stated that when chlorine was used at appropriate doses, it did not cause toxicity in biological tissues. In fact, hypochlorous acid (HOCl), common formulation of chlorine, is used commonly as disinfectant in various areas such as medicine and industry due to its antimicrobial features (Gray et al., 2013).
Applicability of the disinfectants tested in the present study might be evaluated by considering the related literatures. In this context, formaldehyde is the simplest aldehyde compound generated by plants, animals, and humans and it is also produced synthetically and used as disinfectant in medicine, industry and other areas (Zhang, 2018). Use of formaldehyde in A. bisporus cultivation may not create a concern for health as long as not inhaling during its application (Claeys et al., 2009). As for Hydrogen peroxide (H2O2), it is an endogenic reactive oxygen species generated in cells and signalling molecule involved in kinase-driven pathways (Gough & Cotter, 2011). Moreover, it has beneficial traits in maintaining post-harvest quality of button mushroom (Rageh, 2018).
Hypochlorous acid (HOCl) is a natural compound produced against infections in human body and it is a common formulation of chlorine. Chlorine is genereally utilised to get rid of microorganisms in drinking water and swimming pools. With antimicrobial features, it is used as a disinfectant in medical and industrial areas as well (Gray et al., 2013). In addition, it was also reported that hydrogen peroxide and chlorine dioxide (ClO2) could be used at certain doses against green mould in oyster mushroom (Pleurotus spp.) production (Atila, 2020). Sodium hypochlorite is known as liquid bleach used as disinfectant/bleaching agent since the eighteenth century. It is commonly utilised in medicine with its antimicrobial traits. Considering all of these, it could be inferred that the disinfectants tested in the present study might be used as alternative management practices against the green mould in button mushroom cultivation. Moreover, these disinfectants are commercial, cost-effective, and applicable in the in vivo.
In the present study, all the three heat treatments (at 60, 90 and 120 °C for 30 min) induced significant (P < 0.01) yield increases in the treated plots, implying effectiveness of the heat treatments against T. aggressivum f. aggressivum in casing soil. This may have been related to death of conidia of T. aggressivum f. aggressivum as a result of the heat exposures in the treatments. Likewise, in a study, some plant-pathogenic fungi (e.g. Schizophyllum commune, Phytophthora cinnamomi, Phellinus weirii, Ophiostoma novo-ulmi, Fusarium circinatum) did not survive when they were exposed to heat at 55 to 60 °C for 30 min (Ormsby, 2017). In this context, Lee et al. (2014) reported that heat treatments (at 80 °C for 60 to 90 min or at 100 °C for 30 min) of casing soil reduced the diseases (green mould and brown blotch) and caused significant yield increases in button mushroom cultivation. Similarly, Bechara et al. (2009) stated that heat treatments (at 121 °C for 60 and 180 min) of casing soil increased the yield of button mushroom. All these findings are in agreement with the result of the present study.
With regard to the biological efficiency (BE), in the present study, BE ranged from 77.12 to 95.42% among all the controls (inoculated with T. aggressivum f. aggressivum). In this regard, Gea et al. (2013) reported BE values ranging 66.5 to 123.0% in plots inoculated with Lecanicillium fungiola (dry bubble disease of A. bisporus). In another study with the dry bubble disease, Chrysayi-Tokousbalides et al. (2007) found BE values varying from 84.83 to 132.24% in control (inoculated) plots. These findings are consistent with the results of the present study. BE values (87.16 to 105.72%) of majority of the treatments were significantly higher than those of the controls in the present study. In this respect, Chrysayi-Tokousbalides et al. (2007) reported that several fungicides (prochloraz, famoxadone, tebuconazole, trifloxystrobin, mancozeb and carbendazim) applied against the dry bubble disease of A. bisporus caused BE values ranging from 63.28 to 137.20% in treated plots. Considering all of these findings, it may be concluded that the disinfectants and heat treatments inducing BE up to 105.72% in the present study may also indicate efficacy of the treatments tested against T. aggressivum f. aggressivum in casing soil.
In fact, it is hard to find information about management of green mould disease in button mushroom cultivation worldwide. However, to give some examples regarding management of fungal diseases of mushrooms, efficacy of peracetic acid was reported in the management of cobweb disease of button mushroom. In addition, in several in vitro studies, effectiveness of some essential oils against fungal pathogens (Cladobotryum, Mycogone, Lecanicillium) including green mould (T. harzianum and T. aggressivum f. europaeum) were reported (Górski et al., 2010; Đurović-Pejčev et al., 2014), but they were found to be non-selective enough and had negative effects on sporophores of A. bisporus. For example, essential oils from Zatariam ultiflora, Satureja hortensis, Pelargonium roseum and Mentha piperita displayed antifungal activity against A. bisporus. In addition, cost and practising problems of the essential oils in the in vivo made their use in button mushroom cultivation less applicable than disinfictants (Geösel et al., 2014; Mehrparvar et al., 2016).
Conclusion
Since the scarcity of the information regarding management of the green mould (T. aggressivum f. aggressivum) in button mushroom cultivation, the results of this study present new knowledge for mushroom industry. The overall results of the present study suggested that the tested disinfectants (hydrogen peroxide, formaldehyde, sodium hypochlorite and hypochlorous acid) and heat applications (60, 90 and 120 °C) could induce significant yield increases in button mushroom by suppressing growth of T. aggressivum f. aggressivum in casing soil. Thus, these management practices can be used against T. aggressivum f. aggressivum in casing soil in button mushroom cultivation to mitigate the damage caused by the green mould disease.
Data availability
No datasets were generated or analysed during the current study.
References
Atila, F. (2020). Chlorine dioxide as an alternative disinfectant for disinfection of oyster mushroom growing media. The Journal of Horticultural Science and Biotechnology, 95, 121–127. https://doi.org/10.1080/14620316.2019.1634490
Aydoğdu, M., Kurbetli, İ, Kitapçı, A., & Sülü, G. (2020). Aggressiveness of green mould on cultivated mushroom ( Agaricus bisporus ) in Turkey. Journal of Plant Diseases and Protection, 127, 695–708. https://doi.org/10.1007/s41348-020-00328-8
Aydoğdu, M., Sülü, S. M., Kurbetli, İ, & Sülü, G. (2021). In vitro and in vivo biological control of the green mold using different bacteria in button mushroom cultivation. Egyptian Journal of Biological Pest Control, 31, 70. https://doi.org/10.1186/s41938-021-00401-w
Bechara, M. A., Heinemann, P. H., Walker, P. N., Demirci, A., & Romaine, C. P. (2009). Evaluating the addition of activated carbon to heat-treated mushroom casing for grain-based and compost-based substrates. Bioresource Technology,100, 4441–4446. https://doi.org/10.1016/j.biortech.2008.12.064
Berendsen, R. L., Baars, J. J. P., Kalkhove, S. I. C., Lugones, L. G., Wösten, H. A. B., & Bakker, P. A. H. M. (2010). Lecanicillium fungicola: Causal agent of dry bubble disease in white-button mushroom. Molecular Plant Pathology,11, 585–595. https://doi.org/10.1111/j.1364-3703.2010.00627.x
Błaszczyk, L., Popiel, D., Chełkowski, J., Koczyk, G., Samuels, G. J., Sobieralski, K., & Siwulski, M. (2011). Species diversity of Trichoderma in Poland. Microbial Genetics, 52, 233–243. https://doi.org/10.1007/s13353-011-0039-z
Chrysayi-Tokousbalides, M., Kastanias, M. A., Philippoussis, A., & Diamantopoulou, P. (2007). Selective fungitoxicity of famoxadone, tebuconazole and trifloxystrobin between Verticillium Fungicola and Agaricus Bisporus. Crop Protection, 26, 469–475. https://doi.org/10.1016/j.cropro.2006.02.016
Claeys, W., Vleminckx, C., Dubois, A., Huyghebaert, A., Höfte, M., Daenens, P., & Schiffers, B. (2009). Formaldehyde in cultivated mushrooms: A negligible risk for the consumer. Food Additives and Contaminants: Part A, 26, 1265–1272. https://doi.org/10.1080/02652030903081929
Diánez, F., Santos, M., Parra, C., Navarro, M. J., Blanco, R., & Gea, F. J. (2018). Screening of antifungal activity of twelve essential oils against eight pathogenic fungi of vegetables and mushroom. Letters in Applied Microbiology, 67, 400–410. https://doi.org/10.1111/lam.13053
Đurović-Pejčev, R., Potočnik, I., Milijašević-Marčić, S., Todorović, B., Rekanović, E., & Stepanović, M. (2014). Antifungal activity of six plant essential oils from Serbia against Trichoderma aggressivum f. europaeum. Pesticidi i Fitomedicina, 29, 291–297. https://doi.org/10.2298/PIF1404291D
Eryılmaz, M., & Palabıyık, I. M. (2013). Hypochlorous acid-analytical methods and antimicrobial activity. Tropical Journal of Pharmaceutical Research,12, 123–126. https://doi.org/10.4314/tjpr.v12i1.20
Gea, F. J., Carrasco, J., Santos, M., Dianez, F., & Navarro, M. J. (2013). Incidence of lecanicillium fungicola in white-button mushroom ( Agaricus Bisporus ) cultivated with two types of casing soil. Journal of Plant Pathology, 95, 163–166.
Gea, F. J., Navarro, M. J., Santos, M., Diánez, F., & Herraiz-Peñalver, D. (2019). Screening and evaluation of essential oils from Mediterranean aromatic plants against the mushroom cobweb disease, Cladobotryum mycophilum. Agronomy, 9, 656. https://doi.org/10.3390/agronomy9100656
Geösel, A., Szabó, A., Akan, O., & Szarvas, J. (2014). Effect of essential oils on mycopathogens of Agaricus bisporus. 8th International Conference on Mushroom Biology and Mushroom Products (ICMBMP8) 2014p. 534. Retrieved January 4, 2024, from https://www.researchgate.net/publication/268815181_Effect_of_essential_oils_on_mycopathogens_of_Agaricus_bisporus
Górski, R., Sobieralski, K., Siwulski, M., & Góra, K. (2010). Effect of selected natural essential oils on in vitro development of fungus Trichoderma harzianum found in common mushroom ( Agaricus Bisporus ) cultivation. Ecological Chemistry and Engineering, 17, 177–185.
Gough, D., & Cotter, T. (2011). Hydrogen peroxide: A Jekyll and Hyde signalling molecule. Cell Death and Disease, 2, e213. https://doi.org/10.1038/cddis.2011.96
Gray, M. J., Wholey, W. Y., & Jakob, U. (2013). Bacterial responses to reactive chlorine species. Annual Review of Microbiology, 67, 141–160. https://doi.org/10.1146/annurev-micro-102912-142520
Hatvani, L., Antal, Z., Manczinger, L., Szekeres, A., Druzhinina, I. S., Kubicek, C. P., Nagy, A., Nagy, E., Vágvölgyi, C., & Kredics, L. (2007). Green mold diseases of Agaricus and Pleurotus spp. are caused by related but phylogenetically different Trichoderma species. Phytopathology, 97, 532–537. https://doi.org/10.1094/PHYTO-97-4-0532
Hatvani, L., Kredics, L., Allaga, H., Manczinger, L., Vágvölgyi, C., Kuti, K., & Geösel, A. (2017). First report of Trichoderma aggressivum f. aggressivum green mold on Agaricus Bisporus in Europe. Plant Disease, 101, 1052. https://doi.org/10.1094/PDIS-12-16-1783-PDN
Hatvani, L., Sabolić, P., Kocsubé, S., Kredics, L., Czifra, D., Vágvölgyi, C., Kaliterna, J., Ivić, D., Đermić, E., & Kosalec, I. (2012). The first report on mushroom green mould disease in Croatia. Archives of Industrial Hygiene and Toxicology, 63, 481–487. https://doi.org/10.2478/10004-1254-63-2012-2220
Kim, C. S., Shirouzu, T., Nakagiri, A., Sotome, K., Nagasawa, E., & Maekawa, N. (2012). Trichoderma mienum sp. nov., isolated from mushroom farms in Japan. Antonie van Leeuwenhoek,102, 629–641. https://doi.org/10.1007/s10482-012-9758-3
Kosanović, D., Potočnik, I., Duduk, B., Vukojević, J., Stajić, M., Rekanović, E., & Milijašević-Marčić, S. (2013). Trichoderma species on Agaricus bisporus farms in Serbia and their biocontrol. Annals of Applied Biology, 163, 218–230. https://doi.org/10.1111/aab.12048
Kosanović, D., Potočnik, I., Vukojević, J., Stajić, M., Rekanović, E., Stepanović, M., & Todorović, B. (2015). Fungicide sensitivity of Trichoderma spp. from Agaricus Bisporus farms in Serbia. Journal of Environmental Science and Health Part B, 50, 607–613. https://doi.org/10.1080/03601234.2015.1028849
Lee, C. J., Yoo, Y. M., Jhune, C. S., Cheong, J. C., Moon, J. W., Kong, W. S., Suh, J. S., Kim, Y. G., Lee, B. E., & Yoon, M. H. (2014). Effects of microorganism density and mushroom yields according to the sterilization of casing soils at the cultivation of button mushrooms. Journal of Mushrooms,12, 220–225.
Medvediev, D. G., Kerner, A. O., Bondaruk, S. V., & Al-Maali, G. A. (2019). Investigation of cultural features and fungicide resistance of the strains of Cladobotryum mycophilum (Hypocreales, Ascomycota), a causal agent of cobweb disease on button mushroom crops, newly recorded in Ukraine. Ukrainian Botanical Journal,76, 121–131. https://doi.org/10.15407/ukrbotj76.02.121
Mehrparvar, M., Goltapeh, E. M., Safaie, N., Ashkani, S., & Hedesh, R. M. (2016). Antifungal activity of essential oils against mycelial growth of Lecanicillium fungicola var. fungicola and Agaricus bisporus. Industrial Crops and Products, 84, 391–398. https://doi.org/10.1016/j.indcrop.2016.02.012
Mirkhani, F., & Alaei, H. (2015). Species diversity of indigenous Trichoderma from alkaline pistachio soils in Iran. Mycologia Iranica,2, 22–37.
Oh, S. J., Kim, H. K., Kim, H. K., & Fermor, T. R. (2000). Effect of sodium hypochlorite for controlling bacterial blotch on Pleurotus Ostreatus. Mycobiology,28, 123–126. https://doi.org/10.1080/12298093.2000.12015736
Ormsby, M. (2017). Import risk analysis: phytosanitary risks of importing phase III Agaricus bisporus mushroom compost from Northern Europe. MPI Technical Paper No: 2019/16. Retrieved January 4, 2024, from https://www.mpi.govt.nz/dmsdocument/15427-Import-risk-analysis-Phytosanitary-Risks-of-Importing-Phase-III-Agaricus-bisporus-Mushroom-Compost-from-Northern-Europe
Potočnik, I., Rekanović, E., Stepnović, M., Milijašević-Marčić, S., Todorović, B., Nikolić-Bujanović, L., & Čekerevac, M. (2014). Possibility of environmentally-safe casing soil disinfection for control of cobweb disease of button mushroom. Pesticidi i Fitomedicina,29, 283–289.
Qiu, Z., Wu, X., Zhang, J., & Huang, C. (2017). High temperature enhances the ability of Trichoderma Asperellum to infect Pleurotus Ostreatus Mycelia. PLoS ONE, 12, e0187055. https://doi.org/10.1371/journal.pone.0187055
Rageh, M. A. (2018). Maintaining postharvest quality of fresh button mushrooms by application of hydrogen peroxide and antibrowning agent treatments. Annals of Agricultural Science Moshtohor, 56, 721–730.
Santos, T. L. D., Belan, L. L., Zied, D. C., Dias, E. S., & Alves, E. (2017). Essential oils in the control of dry bubble disease in white button mushroom. Ciência Rural,47, e20160780. https://doi.org/10.1590/0103-8478cr20160780
Šantrić, L., Potočnik, I., Radivojević, L., Umiljendić, J. G., Rekanović, E., Duduk, B., & Milijašević-Marčić, S. (2018). Impact of a native Streptomyces flavovirens from mushroom compost on green mold control and yield of Agaricus Bisporus. Journal of Environmental Science and Health Part B, 53, 677–684. https://doi.org/10.1080/03601234.2018.1474559
Sharaf-Eldin, M. A., & Geösel, A. (2016). Efficacy of hydrogen peroxide on postharvest quality of white button mushroom. Egyptian Journal of Horticulture,42, 1–17.
Sharma, S. R., Kumar, S., & Sharma, V. P. (2007). Diseases and competitor moulds of mushrooms and their management (pp. 1–81). Technical Bulletin: National Research Centre for Mushroom (Indian Council of Agricultural Research).
Soković, M., & Van Griensven, L. J. (2006). Antimicrobial activity of essential oils and their components against the three major pathogens of the cultivated button mushroom, Agaricus Bisporus. European Journal of Plant Pathology, 116, 211–224. https://doi.org/10.1007/s10658-006-9053-0
Yang, W., Wang, L., Hu, Q., Pei, F., & Mugambi, M. A. (2019). Identification of bacterial composition in freeze-dried Agaricus Bisporus during storage and the resultant odor deterioration. Frontiers in Microbiology, 10, 349. https://doi.org/10.3389/fmicb.2019.00349
Zhang, L. (2018). Formaldehyde: Exposure, toxicity and health effects. CPI Group: Croydon, Royal Society of Chemistry.
Acknowledgements
I would like to thank SMS Ersanlar mushroom company for providing white (192915 AG, Soc, France) strain of A. bisporus, mushroom compost and casing soil for me.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This research was funded by General Directorate of Agricultural Research and Policies with the project (TAGEM/BSAD/16/7/02/03).
Author information
Authors and Affiliations
Contributions
M A (the corresponding author) did all the work.
Corresponding author
Ethics declarations
Competing interests
The corresponding author declares no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aydoğdu, M. Efficacy of disinfectants and heat treatments against green mould in casing soil and button mushroom (Agaricus bisporus) yield. Phytoparasitica 52, 51 (2024). https://doi.org/10.1007/s12600-024-01168-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12600-024-01168-0