Abstract
Several Oriental and Australian species of Ficus have been introduced outside their native range and planted as ornamentals in urban habitats throughout the Mediterranean. This translocation of plant species has led to the introduction of host-specific insects such as their pollinating fig wasps (Hymenoptera: Agaonidae). Here, the Australian fig wasp Pleistodontes imperialis Saunders, 1882 is reported for the first time from Greece. Partial COI genes were sequenced for specimens sampled in Greece and Cyprus, and they appear to share identical haplotypes. Interestingly, this species-specific wasp not only develops in figs of its usual host, Ficus rubiginosa, but also in figs of Ficus watkinsiana, another Australian species introduced in Greece, which is pollinated by a second agaonid species (Pleistodontes nigriventris Girault, 1915) in its native range. Although no negative economic or environmental impacts have been observed yet, monitoring of alien Ficus spp. in the region is encouraged to prevent their possible establishment in natural habitats.
Similar content being viewed by others
Introduction
During recent centuries, several species of the genus Ficus L. (Moraceae) have been introduced as ornamentals outside their native range, where they have been extensively planted in roadsides, parks, and house gardens (Reyes-Betancort et al., 2013; Speciale et al., 2015; van Noort et al., 2013). Associated mutualist pollinators of the family Agaonidae such as Eupristina verticillata Waterson, 1921 and Pleistodontes imperialis Saunders, 1882, the pollinators of Ficus microcarpa L. and F. rubiginosa Desf. respectively, have followed their host plants and established viable populations in regions far from their native Oriental and Australian natural ranges (Speciale et al., 2015; Wang et al., 2015, b).
Eupristina verticillata, was at first detected in Tunisia (1985), followed by the Canary Islands (Spain) (1989), Italy (1990), Spain (mainland) (1994), Turkey (2011), Malta (2011), Greece (2011–2012), Libya (2011–2012) and Morocco (2010) (Lo Verde et al., 1991; Kobbi et al., 1996; Baez, 1998; Doganlar, 2012; Wang et al., 2015, b). In addition to F. microcarpa, the Port Jackson fig. (F. rubiginosa) has been also introduced and widely planted in the Mediterranean (Haine et al., 2006) and this has led to the concurrent introduction of its pollinator P. imperialis in various sites across the Western Palearctic such as the Canary Islands (Reyes-Betancort et al., 2013), Cyprus (Compton et al., 2020), Israel (Lopez-Vaamonde et al., 2002), Italy (Speciale et al., 2015), France (Rasplus, unpublished), Malta (Mifsud et al., 2012), Portugal (Rasplus, unpublished) and Spain (Kjellberg et al., 2001). Finally, the pollinator of Ficus religiosa, Platyscapa quadraticeps (Mayr, 1885) has followed its host-plant to the Middle Eastern countries of Iraq and Israel (Joseph, 1982; Chen & Chou, 1997).
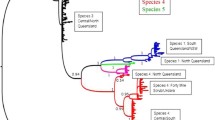
The genus Pleistodontes Saunders (excluding Pleistodontes claviger which is now placed in the genus Platyscapa according to Harrison et al. (2017)), is composed of 25 described species which pollinate Ficus species belonging in the subgenus Spherosuke (=Urostigma) sect. Malvanthera and was revised by Lopez-Vaamonde et al. (2002). Analyses of cytochrome b sequences of P. imperialis associated with F. rubiginosa in Eastern Australia showed deep molecular divergences within this putative species (Haine et al., 2006). P. imperialis was therefore considered to be a complex of four closely related species (Haine et al., 2006). More recent analyses by Darwell et al. (2014) suggested the presence of a fifth species. However, morphological examination of these entities, showed only slight morphological differences (Rasplus, pers. obs).
Until now, there has been no report of the presence of Pleistodontes imperialis in Greece, although it is already widely distributed throughout the Mediterranean. Here, we present the first record of P. imperialis in Greece. Additionally, we provide DNA barcodes for specimens from Greece and Cyprus to facilitate and confirm their identification.
Materials and methods
Study area, sampling and identification
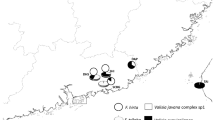
Surveys took place at various urban sites in Southern Greece from April 2021 to September 2022. Mature figs of both F. rubiginosa and F. watkinsiana were collected and stored in polyethylene zip bags for rearing. Additional material was collected from F. rubiginosa in Cyprus for morphological and molecular comparisons. Sampling was also conducted from other fig tree species belonging to subgenus Spherosuke (=Urostigma) sect. Malvanthera (such as F. macrophylla), in search of the pollinator (Fig. 1a-d). Sampled localities are summarized in Table 1 and Fig. 2. Fig tree species were identified using the key of Dixon (2003). Most specimens were stored in 70% ethanol and deposited at the Museum of Zoology of the National and Kapodistrian University of Athens (ZMUA). Fig wasps that emerged were examined under a stereomicroscope following the identification key and species descriptions provided by Lopez-Vaamonde et al. (2002). Specimens were also stored in 90o ethanol for further molecular treatment.
In total, ten specimens were kept in ethanol and sent to the Laboratory of Forest Entomology (Forest Research Institute, Thessaloniki) for molecular screening. DNA was extracted from each specimen individually, using the PureLink™ Genomic DNA mini kit (Invitrogen) following the manufacturers’ protocol. Then, amplification of an approximately 650–700 bp locus was carried out with primers LCO2198 and HCOI1490 (Folmer et al., 1994). Each reaction contained 12.5 μl of DreamTaq DNA Polymerase™ including DreamTaq PCR Master Mix (2X) (ThermoFisher Scientific Baltics UAB, Lithuania), 0.2 μl of each primer (20μΜ), 2 μl of DNA extract and finally ddH2O to the volume of 25 μl. PCR conditions included an initial denaturation step at 94 °C for 1 minutes, followed by 5 cycles at 94 °C for 60s, 45 °C for 90s and 72 °C for 75 s, and then 40 cycles at 94 °C for 60s, 51 °C for 90s and 72 °C for 75 s with a final extension step that lasted 5 minutes at 72 °C. Products were then purified with PureLink ™ PCR Purification Kit (Invitrogen) and sequenced at a commercially available service (Cemia SA, Larissa – Greece). The acquired sequences were visualized with ChromasLite® (Technelysium Pty Ltd) and aligned with CLUSTALX (Thompson et al., 1997). Finally, sequences were compared (blasted) on NCBI GenBank Fig. 2.
Results and discussion
Morphological examination of the sampled specimens confirmed they belong to the P. imperialis species complex (Lopez-Vaamonde et al., 2002). As there is no key to species for this complex, the wasps were subsequently compared to material sequenced by Haine et al. (2006) and Darwell et al. (2014) sent to us by James Cook (Univ. Sydney). They were also compared to specimens previously compared to the paralectotype specimens hosted in NHM London. These morphological analyses confirmed the specimens as belonging to P. imperialis sensu stricto. Blasting the sequences from the Greek specimens on GenBank (NCBI) confirmed our morphological identification of Pleistodontes imperialis as they were 98.9% similar to the only P. imperialis sequence deposited (GQ367882). The specimen from which the GenBank sequence was obtained, was sampled in Western Australia, Rottnest Island (−31.996115.541) on 5.10.2005 by Jousselin E. & Cœur d’acier A. Western Australia is also an area where P. imperialis have been introduced. A close examination of specimens from the same samples and hosted at CBGP, Montpellier (n° JRAS01396_01) confirmed they also belong to P. imperialis s.s. All other sequenced species of Pleistodontes were more distantly related, exhibiting 91.04% and 89.83% similarity with P. nitens (GQ367886) and P. rigisamos (AF200409), respectively.
These results confirmed the species sampled in Greece belong to P. imperialis. This is the first report of P. imperialis in this country. In Greece, the species is distributed at least throughout Attica and Cyclades, whereas material sampled from F. rubiginosa, and F. watkinsiana from Crete, the Dodecanese and the Ionian islands yielded no agaonids. Moreover, material from F. macrophylla throughout southern Greece, lacked pollinators as well.
Although each fig-pollinating Agaonidae species was previously considered to be an obligatory mutualist of a specific Ficus species, recent studies have shown that introduced fig wasps can develop in figs of new partners. This disrupts the obligate association between figs and their pollinators (Bernard et al., 2020; Yu et al., 2021). Moreover, increased sampling of fig tree species and the use of molecular tools have shown numerous Ficus spp. with large distribution that are pollinated by two or more agaonid species and vice versa (Bernard et al., 2020; Darwell et al., 2014; Haine et al., 2006; Machado et al., 2005; Michaloud et al., 1985; Rasplus, 1996).
This was particularly evident in the case of P. imperialis, which was found developing in figs of Ficus watkinsiana F.M.Bailey and Ficus rubra Vahl (Bernard et al., 2020) on the Hawaiian island of Kauai as well as in the Moreton Bay fig Ficus macrophylla Desf. in Italy (Speciale et al., 2015). Interestingly, according to Rønsted et al. (2007), the closest relative of F. rubiginosa is F. watkinsiana, which may partly explain the exchange of its pollinator outside their native range. All these fig tree species have been known to be mutualistic associated with other representatives of the genus Pleistodontes (Lopez-Vaamonde et al., 2002). Previous records of P. imperialis with Ficus obliqua G.Forst. (Bouček, 1988) and Ficus platypoda (Miq.) A.Cunn. ex Miq. (Zammit & Schwarz, 2000) within the species’ native range resulted in fact from misidentifications of F. rubiginosa and therefore were incorrect (Lopez-Vaamonde et al., 2002).
Host switches of pollinators has allowed hybridization events between closely related plant species (Wang et al., 2021). Contrastingly to fig tree hybridization between different Ficus species, there have been no similar signs of hybridism between their mutualistic pollinating fig wasps (Satler et al., 2023; Wang et al., 2021). Non-sympatric fig species of the same section and subgenus (i.e., Urostigma, Urostigma s.s.) have been reported to hybridize, once one of the two species is introduced within the natural distribution of the other, such as F. aurea with F. religiosa in Florida, USA (Ramírez-Benavides, 1994). Given this, the host switch of P. imperialis leading to the hybridization between F. rubiginosa and F. watkinsiana could be possible in areas where these two species have been introduced and co-exist, such as in Cyprus and Greece. Since the hybrid seed production and by extend the establishment of hybrid fig populations is a possible scenario, hybrid seed viability tests would be useful in the future.
The co-occurrence of another widespread fig tree species (F. microcarpa) with its mutualistic pollinator E. verticillata in their introduced areas has allowed the tree to become invasive, with well-known adverse environmental and socioeconomic impacts. Similarly, the presence of P. imperialis throughout the Mediterranean could assist the production of F. rubiginosa seedlings and facilitate its invasiveness. While seedlings of F. microcarpa have been detected in Southern Greece (Galanos, 2015; EK unpublished data) no similar case has been observed regarding F. rubiginosa or F. watkinsiana. Nevertheless, the discovery of seedlings of F. macrophylla in Italy along with the presence of P. imperialis in figs of a nearby older tree (Speciale et al., 2015), signifies the need of further monitoring of both host-plants and their association with P. imperialis in their introduced areas.
Data availability
No datasets were generated or analysed during the current study.
References
Baez, M. (1998). Sobre la presencia del himenóptero polinizador de los “Laureles de Indias” en las Islas Canarias. Boletin de la Asociacion española de Entomologia, 22(1–2), 225.
Bernard, J., Brock, K. C., Tonnell, V., Walsh, S. K., Wenger, J. P., Wolkis, D., & Weiblen, G. D. (2020). New species assemblages disrupt obligatory mutualisms between figs and their pollinators. Frontiers in Ecology and Evolution, 8, 564653. https://doi.org/10.3389/fevo.2020.564653
Bouček, Z. (1988). Australasian Chalcidoidea (Hymenoptera), a biosystematic revision of genera of fourteen families, with a reclassification of species. CAB International
Compton, S. G., Newton, H., Stavrinides, M., & Kaponas, C. (2020). First confirmed records of fig wasps (Hymenoptera: Chalcidoidea) associated with the sycamore fig Ficus sycomorus on a Mediterranean Island. Entomologist’s Gazette, 71, 121–124. https://doi.org/10.31184/G00138894.712.1735
Chen, C. H., Chou, L. Y. (1997). The Blastophagini of Taiwan (Hymenoptera: Agaonidae: Agaoninae). Journal of Taiwan Museum 50(2):145.
Darwell, C., Al-Beidh, S., & Cook, J. (2014). Molecular species delimitation of a symbiotic fig-pollinating wasp species complex reveals extreme deviation from reciprocal partner specificity. BMC Evolutionary Biology, 14, 189. https://doi.org/10.1186/s12862-014-0189-9
Dixon, D. J. (2003). A taxonomic revision of the Australian Ficus species in the section Malvanthera (Ficus subg. Urostigma: Moraceae). Telopea, 10(1), 125–153. https://doi.org/10.7751/telopea20035611
Doganlar, M. (2012). Occurrence of fig wasps (Hymenoptera: Chalcidoidea) in Ficus caria and F. microcarpa in Hatay, Turkey. Turkish Journal of Zoology, 36(5), 721–724. https://doi.org/10.3906/zoo-1111-3
Folmer, O., Black, M., Hoeh, W., Lutz, R., & Vrijenhoek, R. (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology, 3, 294–299
Galanos, C. (2015). The alien flora of terrestrial and marine ecosystems of Rodos island (SEAegean), Greece. Willdenowia, 45, 261–278. https://doi.org/10.3372/wi.45.45211
Haine, E. R., Martin, J., & Cook, J. (2006). Deep mtDNA divergences indicate cryptic species in a fig-pollinating wasp. BMC Evolutionary Biology, 6, 83
Harrison, R., Chong, K. Y., Pham, N. M., Yee, A. T. K., Yeo, C. K., Tan, H. T. W., Rasplus, J. -Y. (2017). Pollination of Ficus elastica: India rubber re-establishes sexual reproduction in Singapore. Scientific Reports, 7, 11616. https://doi.org/10.1038/s41598-017-09873-z
Joseph, K. J. (1982). New record of the fig wasp Blastophaga quadraticeps Mayr with remarks on the introduction of this insect to Iraq and on the dispersal of its host plant Ficus religiosa L. Bulletin of Basrah Natural History Museum, 5,101–107.
Kjellberg, F., Jousselin, E., Bronstein, J. L., Patel, A., Yokoyama, J., & Rasplus, J.-Y. (2001). Pollination mode in fig wasps: The predictive power of correlated traits. Proceedings of the Royal Society: Biological Sciences, 268, 1113–1121
Kobbi, M., Chaieb, M., Edelin, C., & Michaloud, G. (1996). Relationship between a mutualist and a parasite of the laurel fig, Ficus microcarpa L. Canadian Journal of Zoology, 74, 1831–1833
Lo Verde, G., Porcelli, F., Sinacori, A. (1991). Presenza di Parapristina verticillata (Waterst.) e Odontofroggatia galili Wiebes (Hymenoptera: Chalcidoidea Agaonidae) in Sicilia. Atti del XVI Congresso Nazionale Italiano di Entomologia, 139–143.
Lopez-Vaamonde, C., Dixon, D. J., Cook, J. M., & Rasplus, J.-Y. (2002). Revision of the Australian species of Pleistodontes (Hymenoptera: Agaonidae) fig-pollinating wasps and their host-plant associations. Zoological Journal of the Linnean Society, 136(4), 637–683. https://doi.org/10.1046/j.1096-3642.2002.00040.x
Machado, C. A., Robbins, N., Gilbert, M. T. P., & Herre, E. A. (2005). Critical review of host specificity and its coevolutionary implications in the fig/fig-wasp mutualism. Proceedings of the National Academy of Sciences of the United States of America, 102, 6558–6565
Michaloud, G., Michaloud-Pelletier, S., Wiebes, J. T., & Berg, C. C. (1985). The co-occurrence of two pollinating species of fig wasp and one species of fig. Proceedings of the Koninldijke Nederlandse Akademie van Wetenschappen (C), 88, 93–119
Mifsud, D., Falzon, A., Malumphy, C., Delillo, E., Vovlas, N., & Porcelli, F. (2012). On some arthropods associated with Ficus species (Moraceae) in the Maltese islands. Bulletin of the Entomological Society of Malta, 5, 5–34
Ramírez-Benavides, W. (1994). Hybridization of Ficus religiosa with F. Septica and F. Aurea (Moraceae). Revista de Biología Tropical, 42, 339–342
Rasplus, J.-Y. (1996). The one-to-one species-specificity of the Ficus-Agaoninae mutualism: How casual? In L. J. G. Van der Maesen, X. M. van der Burgt, & J. M. van Medenbach de Rooy (Eds.), The Biodiversity of African plants (pp. 639–649). Kluwer Academic Publishers
Reyes-Betancort, A. R., Hernández-Suárez, E., & Polaszek, A. (2013). First record of Pleistodontes imperialis Saunders, 1882 (Hymenoptera: Chalcidoidea, Agaonidae) in the Canary Islands. Boletín de la Asociación española de Entomología, 37(1–2), 1–3
Rønsted, N., Weiblen, G., Clement, W., Zerega, N., & Savolainen, V. (2007). Reconstructing the phylogeny of figs (Ficus, Moraceae) to reveal the history of the fig pollination mutualism. Symbiosis, 45(1), 45–55
Satler, J. D., Herre, E. A., Heath, T. A., Machado, C. A., Gómez Zúñiga, A., Jandér, K. C., Eaton, D. A. R., & Nason, J. D. (2023). Pollinator and host sharing lead to hybridization and introgression in Panamanian free-standing figs, but not in their pollinator wasps. Ecology and Evolution, 13, e9673. https://doi.org/10.1002/ece3.9673
Speciale, M., Cerasa, G., & Lo Verde, G. (2015). First record in Europe of seedling of Ficus macrophylla f. columnaris (Moraceae) and of its pollinating wasp Pleistodontes cf. imperialis (Chalcidoidea Agaonidae). Il Naturalista Siciliano, S, 39(2), 399–406
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., & Higguns, D. G. (1997). The CLUSTAL_X windows Interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research, 24, 4876–4882
van Noort, S., Wang, R., & Compton, S. G. (2013). Fig wasps (Hymenoptera: Chalcidoidea: Agaonidae, Pteromalidae) associated with Asian fig trees (Ficus, Moraceae) in southern Africa: Asian followers and African colonists. African Invertebrates, 54(2), 381–400. https://doi.org/10.5733/afin.054.0208
Wang, G., Zhang, X., Herre, E. A., McKey, D., Machado, C. A., Yu, W.-B., Cannon, C. H., Arnold, M. L., Pereira, R. A., Ming, R., Liu, Y.-F., Wang, Y., Ma, D., & Chen, J. (2021). Genomic evidence of prevalent hybridization throughout the evolutionary history of the fig-wasp pollination mutualism. Nature Communications, 12, 718. https://doi.org/10.1038/s41467-021-20957-3
Wang, R., Aylwin, R., Barwell, L., Chen, X. Y., Chen, Y., Chou, L. S., Cobb, J., Collette, D., Craine, L., Giblin-Davis, R. M., Ghana, S., Harper, M., Harrison, R. D., McPherson, J. R., Peng, Y. Q., Pereira, R. A. S., Reyes-Betancort, A., Rodriguez, L. J. V., Strange, E., … Compton, S. G. (2015). The fig wasp followers and colonists of a widely introduced fig tree, Ficus microcarpa. Insect Conservation and Diversity, 8, 322–336. https://doi.org/10.1111/icad.12111
Wang, R., Aylwin, R., Cobb, J., Craine, L., Ghana, S., Reyes-Betancort, J., Quinnell, R., & Compton, S. G. (2015). The impact of fig wasps (Chalcidoidea), new to the Mediterranean, on reproduction of an invasive fig tree Ficus microcarpa (Moraceae) and their potential for its biological control. Biological Control, 81, 21–30. https://doi.org/10.1016/j.biocontrol.2014.11.004
Yu, H., Liao, Y., Cheng, Y., Jia, Y., & Compton, S. (2021). More examples of breakdown the 1:1 partner specificity between figs and fig wasps. Botanical Studies, 62, 15. https://doi.org/10.1186/s40529-021-00323-8
Zammit, J., & Schwarz, M. P. (2000). Intersexual sibling interactions and male benevolence in a fig wasp. Animal Behaviour, 60(5), 695–701
Acknowledgements
We would like to thank the Hellenic Entomological Society for funding part of Mr. Evangelos Koutsoukos’ MSc thesis, which indirectly contributed to the present research. Mr. Evangelos Koutsoukos acknowledges COST Action CA17122 – Alien CSI, supported by COST (European Cooperation in Science and Technology), www.cost.eu. We are also grateful to the UK Government through Darwin Plus for funding Mr. Evangelos Koutsoukos under the project DPLUS202, and Mr. Jakovos Demetriou under the project DPLUS124, which contributed to this present research. We warmly thank Dr. James Cook (Univ. Sydney) for loan of material sequenced in his studies.
Funding
Open access funding provided by HEAL-Link Greece. This article/publication is based upon work from COST Action CA17122 – Alien CSI, supported by COST (European Cooperation in Science and Technology), www.cost.eu. Jakovos Demetriou acknowledges the UK Government through Darwin Plus (DPLUS0124), for funding his material surveys. Evangelos Koutsoukos acknowledges the UK Government through Darwin Plus (DPLUS0202), for funding his material surveys.
Author information
Authors and Affiliations
Contributions
Conceptualization: Evangelos Koutsoukos, Jakovos Demetriou; Methodology: Evangelos Koutsoukos, Stephen Compton, Dimitrios Avtzis, Jean-Yves Rasplus; Formal analysis and investigation: Evangelos Koutsoukos, Jakovos Demetriou, Angeliki Martinou, Stephen Compton, Dimitrios Avtzis, Jean-Yves Rasplus; Writing - original draft preparation: Evangelos Koutsoukos, Jakovos Demetriou, Stephen Compton, Dimitrios Avtzis; Writing - review and editing: Evangelos Koutsoukos, Stephen Compton, Angeliki Martinou, Dimitrios Avtzis, Jean-Yves Rasplus; Resources: Evangelos Koutsoukos, Jakovos Demetriou, Dimitrios Avtzis, Jean-Yves Rasplus; Supervision: Stephen Compton, Jean Yves-Rasplus.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koutsoukos, E., Demetriou, J., Martinou, A.F. et al. Playing both fig sides: the presence and host-switch of Pleistodontes imperialis (Hymenoptera: Agaonidae) is confirmed in Greece and Cyprus. Phytoparasitica 52, 39 (2024). https://doi.org/10.1007/s12600-024-01158-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12600-024-01158-2