Abstract
Due to the damaging effects of root-knot nematodes on crops and the dangerous effects of chemical nematicides on both people and the environment in Egypt, the purpose of this study was to assess the power of polysaccharides and polyphenol extracts as well as their nano-forms from marine algae (Laurencia papillosa and Dilophys fasciola) used as eco-friendly alternatives for the control of Meloidogyne incognita. The nano-forms of algal extracts efficiently suppressed M. incognita egg hatching and increased juvenile mortality compared to the control. The tested treatments effectively decreased galls and egg masses of tomato roots compared to the control in the field. Dilophys fasciola extract and its nano-form showed promising nematicidal activity compared to L. papillosa extract. Generally, algal treatments boosted tomato plant defense system against M. incognita by triggering the production of some biochemical constituents such as phenolic compounds, polyphenol oxidase and chitinase enzymes. Consequently, the productivity and quality parameters of tomato fruits significantly increased.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Egypt, tomato (Solanum lycopersicum) is the most widely planted and utilized vegetable crop. Tomato yield loss due to root-knot nematodes was between 24 and 38% (El-Nagdi et al., 2019; Ong et al., 2020). Plant nematode control involves various chemical nematicides that have environmental consequences for humans and animals. The usage of such chemical substances is subject to numerous cautions and rules (Abd-Elgawad & Askary, 2015; Saad et al., 2019). Root-knot nematodes are economically important pest and obligate plant parasites with a high degree of adaptation (Archidona-Yuste et al., 2018; Khalil et al., 2022; Niu et al., 2020). They cause yield losses in many plant species, particularly within tropical and subtropical agriculture (Chen and Song 2021; Kayani et al., 2018).
In recent years, commercial bio-control preparations against root-knot nematodes have been developed as eco-friendly and safer for humans. Organic algae derivatives are an innovative way to decrease the utilization of dangerous nematicides and have a substantial impact on controlling and inhibiting plant nematode infestation (Hamouda & El-Ansary, 2017; El-Ansary & Hamouda, 2014) and prevent the usage of dangerous chemicals harming ecosystems. Marine algae are rich in compounds which have been investigated as biocidal and medicinal agents (Blunden, 1991; Craigie, 2011). Some of the substances including agar, carrageenan, carotenes, alginic acids, bromine-containing alkaloids, acetogenins, and phenolic compounds, showed a pesticidal effect (Asimakis et al., 2022; Fenical, 1982; Ibañez & Cifuentes, 2013; Wang et al., 2014). In addition, nematode resistance of vegetables, fruits, and other crops has been stimulated by the application of marine algae (Ara et al., 2002; Silva et al., 2004; Wu et al., 1997).
Phenolic compounds play a role in plant defense since they have great potential against nematodes (Ohri & Pannu, 2010). Rajesh et al. (1985) studied the nematicidal activity of phenolic compounds and their effect on hatching of Meloidogyne incognita eggs. Trans-cinnamic acid had the highest nematicidal activity, followed by pyrogallol, 2-hydroxynapthoic acid, and ethyl gallate. Ferulic acid and caffeic acid were also highly effective against M. incognita juveniles. Numerous enzymes, such as polyphenol oxidase, protease, and chitinase, have been related to nematode reduction, activating plant defense and their function in plants' defense mechanisms (Mohammad et al., 2022; Ojaghian et al., 2014).
On the other hand, the widespread application and beneficial use of nanotechnology in a variety of applications have triggered the interest of agricultural experts, who are working to modernize the agricultural sector through Agri-nanotechnology (Worrall et al., 2018), including improved nutrient uptake, improved plant pathogen control, long-term absorption of agricultural products, and enhanced plant development (Nikoo et al., 2014; Prasad et al., 2017a, b; Singh et al., 2021; Zhang et al., 2008). The creation of nano-particles by biological systems such as microbes, polysaccharides, plants, and algae is gaining popularity due to their ease of use and environmental friendliness when compared to physical and chemical methods, showing increased toxicity against the targeted pests (Abdellatif et al., 2016; Benelli et al., 2017).
In addition, nano-sized formulations can efficiently deliver the active components into pest and minimise the active doses, control the release of active ingredients and effectively prolong the control period (El Wakeil et al., 2017). Therefore, this research aimed to develop sustainable bio-control approaches by employing marine algae extracts and their nano-forms to protect tomato plants against nematode infestation and improve the productivity and quality of tomatoes under protected cultivation.
Materials and Methods
Dilophys fasciola (Roth) was collected from the Mediterranean Sea in Marsa Matrouh governorate in August 2019, and Laurencia papillosa (C. Agardh) was collected from the Suez Gulf'—Ein Al-Sokhna region in April 2020. The collected material was identified by Dr. Rohiya Abdel-Latif, professor in the Department of Botany in the Faculty of Science et al.-Azhar University in Egypt. All algal samples were washed in water and dried in shade. The dried samples were ground in a grinder (Braun) for approximately then passed through a sieve with a mesh aperture of 600 µm in diameter, and then kept at room temperature in glass containers until future analysis.
Ten infested tomato roots were collected from the Experimental and Production Station of the National Research Centre, El-Noubaria region, Beheira Governorate, north Egypt. Meloidogyne incognita infested roots were identified through light microscopic examination of the female’s perennial pattern according to Moens et al. (2009). The infested roots were cut into 2 cm-long pieces and incubated in water at room temperature for hatching. Active second-stage juveniles (J2S) were collected and maintained at the Plant Pathology Department, National Research Centre, as a source of M. incognita eggs and juveniles.
Preparation of algal extracts
Dried seaweed powder of L. papillosa (1 kg) was heated in distilled water (1:3 w:v) at 90 °C for 12 h. This process repeated three times. The mixture was filtered through Whatman No. 54 filter paper (diameter 90 mm, pore size 22 µm) and poured into 3 L of absolute ethanol to precipitate the polysaccharides. The obtained precipitate was collected by centrifugation at 10,000 rpm and dried at 60 °C (Abdelhafez et al., 2022). The dried polysaccharide extract was packed and kept frozen (-20 °C) for further experimental analysis.
Dilophys fasciola powder (1 kg) was extracted with methanol (1:3 w:v) at room temperature overnight. This process repeated three times to ensure maximum efficiency in the extraction process. The mixture was then filtered through Whatman filter paper No. 54 (diameter 90 mm, pore size 22 µm) and evaporated under vacuum at 40 °C using a rotary evaporator. The dried extract was packed and kept frozen (-20 °C) for further experimental analysis.
HPLC analysis of D. fasciola extract
Phenolic compounds of D. fasciola were identified using HPLC (Agilent 1100 series coupled with UV–Vis detector (G1315B) and degasser (G1322A) according to Ben-Hammouda et al. (1995).The sample (5 μL) was injected with an Agilent 1100 series automatic sampler. Chromatographic separations were performed on a ZORBAX-Eclipse XDB- C18 column (4.6 × 250 mm, particle size 5 μm). A constant flow rate of 1 mL/min was used with a mobile phase which consisted of (A) 0.5% acetic acid in distilled water at pH 2.65; and (B) 0.5% acetic acid in 99.5% acetonitrile. The elution gradient was linear starting with (a) and ending with (b) over 50 min, using a UV detector set to wavelength 280 nm. HPLC analysis of the algal extract was carried out in triplicate and the phenolic compounds were identified by comparing the relative retention time and spectral matching with the reference materials, Gallic acid, pyrogallol, 4-amino- benzoic acid, ferulic acid, catechin, chlorogenic acid, catechol, ellagic acid, caffeine, p-hydroxy benzoic acid, caffeic acid, vanillic acid, p-coumaric acid, salicylic acid, naringin, hesperidin, apigenin-6-arabinose-8-galactose, apigenin-6-rhamnose-8-glucose, rutin, rosmarinic acid, kaempferol-3-(2- p-coumaroyl) glucose, apigenin-7-glucose, naringenin, quercetin, kaempferol and apigenin were purchased from Sigma–Aldrich, Inc. (Louis, USA).
For quantification of phenolic compounds, increasing concentrations of each standard in methanol in the range of 0.25–1 mg/mL were prepared. The corresponding calibration curves fitted with the trend line equation were obtained by plotting the concentration as the independent (x) and the peak area as the dependent (y) variables. The concentration of each phenolic compound was calculated using the corresponding trend line equation. The samples were analyzed in the Central Lab of the National Research Center-Cairo -Egypt (Chromatography Lab). These parameters and the whole methodology parameters are owned by the lab team.
Preparation of algal extracts nano-suspension
Two algae extracts (L. Papillosa and D. fasciola) nano-forms were prepared utilizing the micro-mini-emulsion polymerization technique outlined by Youssef and Abdelmegeed (2021) and Zhang et al. (2008).The process was used to create the polyethylene glycol (PEG) loaded nano-emulsions. The algal extracts were diluted and dropped into a 1:1 (v/v) ratio of polyethylene glycol solution (3% v/v) at room temperature while being continuously mechanically stirred. An ultrasonic cleaning set (model WUC-DO3H, 290W, 60 Hz) was used to sonicate the suspension for 60 min before a high-energy ultra-sonication probe was used for 3 min (model VCX750, 750W, 20 kHz). Overnight, the loaded nanocapsule suspension was changed.
Transmission Electron Microscopy (TEM) of nano-forms
Two nano-capsule suspensions were placed in a copper grid after being diluted with distilled water that had been coated with carbon before being studied and photographed at high magnification (20000X). Transmission Electron Microscopy (TEM) was used to examine the morphological shapes of the prepared nano-forms (Jeol, JEM-2100).
Preparation of M. incognita eggs and juveniles
Ten tomato roots from pure culture were extracted by sodium hypochlorite (0.5%) for three min. The liquid egg suspension was passed through a 500-mesh sieve. The number of extracted eggs was 10,000 obtained from egg masses (Hussey & Barker, 1973).The obtained eggs (10,000) were divided into two parts: one part was used for egg hatchability, and the other was incubated in water at a temperature of 25 ± 5 °C for hatching. The newly hatched J2S were used for J2 mortality bioassay.
Effect of algal extract and their nano-forms on M. incognita egg hatchability
The activity of algal polyphenols (APP) and algal polysaccharides (APS) at concentrations of 12.5 (APP1 and APS1) and 6.25 g/100 mL (APP2 and APS2) as well as their nano-forms (ANPS1, ANPS2, ANPP1, and ANPP2) against M. incognita egg hatching was evaluated according to Yang et al. (2016). One mL of M. incognita egg suspension containing about 100 ± 5 eggs was added to a test tube containing 4 mL of algal extract. Distilled water with egg suspensions was used as the control. All test tubes were maintained at room temperature (25 ± 5 °C) for seven days of exposure. Hatched eggs were counted under a light microscope using a Hawksley slide. All treatments were done in five biological replicates, and the relative suppression rate was calculated as follows
Effect of algal extract and their nano-forms on M. incognita juvenile mortality
The effects of algal extracts and their nano-forms on the mortality of M. incognita juveniles were examined. In a test tube containing 4 mL of algal extract (APS1, APS2, ANPS1, ANPS2, APP1, APP2, ANPP1, and ANPP2) and 1 mL of nematode suspension containing 100 ± 5 J2s were added. Plain water with J2 suspensions was used as the control. The tubes were incubated at 25 °C ± 5 °C and the number of deceased juveniles was counted using a light microscope and a Hawksley counting slide after 24 and 48 h of exposure. Nematodes were considered to be alive if they moved or assumed a winding shape, and lifeless if they remained still after being touched with a fire needle (Mukhtar et al., 2013). Dead nematode count has been annotated following two exposure times (24 and 48 h) and the percentage of juvenile mortality was calculated according to Abbott's Formula (1925):
After 48 h of exposure, the juveniles were rinsed with distilled water to reverse the effects of the treatments and then transferred to distilled water for a further 24 h, where after recovery percentages were calculated.
Greenhouse experiment
Soil analysis
The experiment was carried out in an infested greenhouse at the Experimental and Production Station of the National Research Centre, El-Noubaria region, Beheira Governorate, north of Egypt. Physical and chemical properties as well as the initial population of M. incognita of the greenhouse soil were examined before transplanting. The soil was sandy (73.86% sand, 6.78% clay and 19.36% silt, pH 8.09, EC 2.49 d.sm−1). The initial population of M. incognita was estimated to be 780 J2/250 mL soil.
Cultivation and treatment of seedlings
Tomato plants (Solanum lycopersicum Mill. cv. CH7) were transplanted into the greenhouse in September 2020 and in the 2021 seasons. Complete randomized lock designs were used to organize the experiment with five replicates for each block. All agriculture practices were performed as recommended by Egyptian Ministry of Agriculture and Land Reclamation, for tomato cultivation. 200 units of N2 60 units of P2O5, and 100 units of K2O/acre were used to fertilize the plants. After three days of cultivation, the treatments (Table 1) were applied at a rate of 100 mL/seedling as a soil drench into holes created around the plants.
Estimation of biochemical parameters
Biochemical parameters in terms of enzyme activity (chitinase and polyphenol oxidase), total phenol content and total sugar content were estimated at one month after the treatment.
For total phenol and sugar content, fresh leaves (1 g) from each treatment were homogenized in 10 mL of 80% methanol and stirred for 15 min at 70 °C. Total phenolic content was determined using Folin–Ciocalteu method according to Saikia et al. (2016). The results were expressed as milligrams of catechol equivalent per 100 g (mg CE/100 g). Sugar content was estimated as described in the procedure of Dubios et al. (1956).
To determine enzymatic activities, crude enzyme extract was prepared according to McCord and Fridovich(1969) by homogenizing 1 g of chopped leaves from each treatment in 40 ml of phosphate buffer. The extracts were centrifuged at 10,000 rpm for 10 min at 4 °C. Chitinase activity was assessed using the artificial substrate 4-nitrophenyl-N-acetyl-β-D-glucosaminide (0.1%) in 0.05 M phosphate buffer at pH 5.8 according to Rustiguel (2012).The activity of polyphenol oxidase was measured according to Vamos-Vigyazo and Nadudvari-Markus (1982) using 0.5 mL of crude enzyme extract and 1 mL of catechol (0.05 M) as the substrate in 2.5 mL of phosphate buffer (0.1 M, pH 7.0). The absorbance was measured at a consistent period of 30 s at 540 nm.
Growth parameters
Sixty-five days after transplantation, five plants were randomly chosen to estimate the vegetative growth parameters (number of leaves, branch count and plant height), number of clusters, number of fruit weight, fruit yield per plant, and fruit yield per acre.
Fruit quality parameters
In the mid of the fruit harvest, five tomato plants were randomly selected from each treatment and the average of fruit diameter (cm) and weight (g) were determined. Total soluble solids (TSS) were measured in fruit juice using a hand refractometer according to AOAC (2012). The total phenolic content of tomato fruits was determined using the Folin–Ciocalteau method according to Saikia et al. (2016). Lycopene within tomato fruits was extracted according to Beerh and Siddappa (1959) and the absorbance of the obtained extract was measured at 503 nm. The lycopene content of each sample was then calculated according to Fish et al. (2002). The colour parameters L*, a*, and b* values of tomato fruits, which represent light–dark spectrum with a range from 0 (black) to 100 (white), the green–red spectrum with a range from—60 (green) to + 60 ( red) and the blue-yellow spectrum with a range from—60 (blue) to + 60 (yellow) dimensions, respectively were recorded using Hunter Lab with the CIE colour scale (Hunter, Lab scan XE).
Sensory evaluation of tomato fruits
The flavour, pericarp colour, internal colour, and texture of tomato samples were assessed by ten panelists according to Araujo et al. (2014). A piece of tomato for each fruit sample was introduced to the panelists in a coded white plastic cup. The 7-point hedonic scale, which includes seven classes: 1 (extremely disliked), 2 (very disliked), 3 (disliked), 4 (neither liked nor disliked), 5 (liked), 6 (highly liked), and 7 (extremely liked), was used to grad the samples.
Nematode damage of tomato root system
After carefully washing the root with tap water, the total number of galls and egg mass per 5 g of root was measured and classified on a scale from 0 to 10 as described by Sharma et al. (1994) and Barker (1985).
Statistical analysis
Data were subjected to analysis of variance (ANOVA) and significant means separated by Duncan's Multiple Range Test (DMRT) at p ≤ 0.05 levels using the Computer Software Statistical Package (CO-STATE) User Manual, Version 3.03, Barkley Co., USA. The average of replicates from two seasons was used to represent the means (As a two-season analysis combined).
Results
Phenolic and flavonoid profile of D. fasciola extract
HPLC analysis showed that the methanolic extract of D. fasciola was composed of twenty-six phenolic and flavonoid compounds. The most abundant phenolic compounds were ellagic acid (170.56 µg/100 mL) followed by pyrogallol (107.07 µg/100 mL) and chlorogenic acid (63.09 µg/100 mL), while gallic acid (2.13 μg/100 mL) presented less abundant (Table 2).
Morphological shapes of algal nano polysaccharides and nano polyphenols.
The developed nano-capsules of L. papillosa polysaccharides and D. fasciola polyphenol compounds were spherical with smooth surfaces and their particle sizes varied between 50 and 100 nm (Fig. 1). In the micro-mini-emulsion polymerization technique, the core-and-shell capsules were developed due to continuous mechanical stirring. PEG represents the outer shell, and the algal extract represents the inner core.
Nematicidal activity of algal extracts under laboratory conditions
Suppression of M. incognita egg hatching
The suppression activity of L. papillosa polysaccharides and D. fasciola polyphenol and their nano-forms at concentrations of 12.5 and 6.25% respectively, against M. incognita egg hatching is presented in Table 3. Data show that both marine algal extracts significantly (p ≤ 0.05) suppressed M. incognita eggs hatching. APP extract was more effective against M. incognita egg hatching than APS extract. By applying D. fasciola extract, APP1 and APP2 seem to work better, reaching suppression rates of 71.43 and 58.16% respectively, compared to 67.35 and 53.06% for APS1 and APS2. The nano-forms of both extracts (ANPP and ANPS) outperformed their original forms. ANPP1 achieved the highest suppression of M. incognita eggs followed by the ANPS1, which yielded 87.76 and 85.71%, respectively. Generally, D. fasciola extract and its nano-form produced the best suppression of egg hatching.
Mortality of M. incognita J2
The effect of L. papillosa polysaccharides and D. fasciola polyphenols and their nano-forms on M. incognita J2 mortality are presented in Table 4. Data show that both algal extracts significantly (p ≤ 0.05) increased the mortality of J2s. Moreover, the mortality bioassay revealed a positive relationship between the J2 mortality, the dose of treatment, and the time of exposure for both APS and APP and their nano-forms. ANPS1 treatment achieved 100.0% mortality compared to 82.5% for APS1 treatment. Also, the nano-form of D. fasciola extracts was more effective than the original extract. M.incognita J2 reached 100.0 and 85.5% mortality for ANPP1 and APP1 treatments, respectively. The extract of D. fasciola and its nano-form produced higher M. incognita J2 mortality than L. papillosa extract and its nano-form.
Field experiments
Effect of algal extracts and their nano-forms on the biochemical parameters of tomato plants.
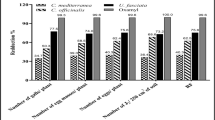
Figure 2 shows the total phenol and sugar contents as well as polyphenol oxidase (PPO) and chitinase activities of tomato plants grown in M. incognita infested sandy soil and treated with algal polysaccharides and polyphenolic compounds and their nano-forms. Algal treatments were very effective in increasing the phenolic and sugar contents of tomato leaves compared to the untreated control and Vydate treatments. The figure highlights that the values of phenolic content increased in a dose-dependent manner in tomato plants treated by algal extracts. Among the preceding treatments, ANPS1 treatment was the most effective in boosting the phenolic content of tomato leaves, followed by ANPP1 and ANPP2, respectively. Regarding the sugar content, the algal treatments effectively boosted its levels in the leaves of tomato plants. ANPS3 treatment had the best effect on the content of total sugar.
Effect of algal extracts and their nano-forms on biochemical parameters of tomato plants. Error bars are SD of the means. *For the full names of the treatments see Table 1 in the Material and Methods section
According to Fig. 2, the activity of PPO enzyme showed different trends. It slightly decreased in tomato plants treated with APS and APP and their nano-forms at the concentrations of 6.25 and 3.12% compared to Vydate application and untreated control. By contrast, the activity of PPO increased in tomato plants treated with APS and APP and their nano-forms at a concentration of 12.5%. The outcomes showed that tomato plants treated with ANPP1 had the highest PPO activity, while those treated with APS3 had the lowest PPO activity. On the contrary, chitinase activity increased in tomato plants treated with algal extracts and their nano-form at all tested concentrations compared to the untreated control. However, the increase in chitinase activity was inversely proportional to the concentration of both D. fasciola and L. papillosa extracts. ANPP3 had the highest chitinase activity compared to the other treatments.
Effect of algal extracts and their nano-forms on the vegetative growth of tomato plants
The vegetative growth parameters of tomato plants grown in sandy soil infested with nematode are presented in Table 5 Plant length, number of branches per plant, number of leaves per plant and fresh weight of leaves per plant were significantly (p ≤ 0.05) increased in the treated plant compared to the untreated control. All algal treatments increased the vegetative growth parameters of tomato plants in a dose-dependent manner. D. fasciola extract and its nano-form outperformed L. papillosa extract and its nano-form in increasing the vegetative growth of the tomato plants. The effect of algal treatments on the vegetative growth parameters can be arranged in ascending order as APS < APP < ANPS < ANPP. The nano-forms of algal extracts were more effective in promoting vegetative growth characteristics of the tomato plants compared to the crude extracts. For instance, tomato plants treated with ANPP1 had the highest values of plant length, number of branches per plant, number of leaves per plant and fresh weight of leaves per plant. These parameters increased by 31.71, 81.81, 103.47, and 203.77%, respectively, compared to the plants of the untreated control.
Effect of algal extracts and their nano-forms on the productivity of tomato plants
Data in Table 6 show the effect of L. papillosa and D. fasciola extracts and their nano-forms on the flowering and fruit yield of tomato plants compared to Vydate application and untreated control. In terms of increasing the metrics of blooming and fruit yield, D. fasciola extract and its nano-formulation outperformed L. papillosa and its nano-form. The nano-forms were more effective than the original extracts in promoting flowering and fruit production. In general, the results revealed that ANPP1 application outperformed the other treatments with significant differences, where cluster count, fruit count, fruit yield (ton/acre, and fruits per plant) increased by 211.76, 467.96, 73.43 and 98.26%, respectively, compared to the untreated control.
Effect of algal extracts and their nano-forms on fruit quality parameters
Data in Table 7 show the impact of algal polysaccharides, polyphenol compounds, and their nano-forms on tomato fruit quality compared to Vydate application and untreated control. Algal treatments significantly enhanced the physicochemical characteristics of tomato fruits. The highest pH, total phenols, lycopene concentration, and redness value (a*) were found in tomato plants treated with ANPP1, measuring 4.05 pH, 39.69 mg/100 g, 164.82 mg/kg, and 32.05, respectively. The TSS values of tomato fruits ranged in a narrow range between 3.95 and 2.30. The highest TSS value was recorded by the fruit of tomato plants treated with Vydate. Moreover, the colour parameters for lightness (L*) and yellowness (b*) fluctuated within a small range, between 40.28—38.24 and 26.72—23.081, respectively.
Effect of algal extracts and their nano-forms on organoleptic properties of tomato fruits
As indicated in Table 8, the sensory qualities of tomato fruits in terms of appearance, pericarp colour, internal colour, texture, and taste were examined concerning algal polysaccharides, phenolic compounds, and their nano-forms. The acquired data demonstrated that, except for taste, the control received the lowest score for the examined properties. The fruits of tomato treated with ANPP1 received the best marks for appearance (7.0), pericarp colour (6.6), internal colour (6.2), and texture (6.2), while the untreated control received the highest taste score (6.2).
Effect of algal treatments on nematodes on infested tomato plants
The effect of algal polysaccharides and polyphenolic compounds and their nano-form treatments on M. incognita J2 number in the soil as well as on the gall and egg-mass number in tomato roots were recorded as presented in Table 9. In general, all treatments significantly (p ≤ 0.05) reduced M. incognita J2s in the soil and gall and egg-mass number in tomato roots. Furthermore, except for APS1-3 and APP3 treatments, the nematode parameters in the soil and tomato plants treated with algal extracts were significantly decreased compared to Vydate treatment. Dilophys fasciola extract and its nano-form were more effective in reducing the nematode parameters than L. papillosa and its nano-form. The decrease of nematode parameter had a positive significant relationship with the applied concentrations.
The nano-forms showed higher nematicidal activity against J2s, gall and egg-mass number compared to the original extracts. For instance, Vydate, APS1, and ANPS1 treatments achieved 70.91, 69.59 and 94.74% reduction of the J2 number in the soil, respectively. Similarly, these treatments decreased gall formation by 59.31, 56.28 and 82.59% respectively. Reductions in egg-mass number yielded 54.40, 47.05 and 64.70% respectively. Application of D. fasciola extract (APP1) and its nano-form (ANPP1) reduced J2 in the soil by 78.80 and 95.61%, respectively. The same treatments reduced the galls and egg-masses in 5 g tomato roots by 65.521 and 61.20% for APP1 and 81.98 and 86.76% for ANPP1, respectively. As well, the nematode indexes of J2 numbers, galls and egg masses recorded the same trend at the lower concentrations. Generally, the results revealed a positive relationship trend between the reduction of nematode parameters and the applied concentrations and its nano-forms (Table 9).
Discussion
The application of green bio-control of parasitic nematodes as an alternative strategy for chemical nematicides is one of the great concerns of modern agriculture systems worldwide. In this context, the extensive studies on seaweeds have led to the discovery of an impressive array of bio-pesticides. However, among the huge numbers of marine algae and the myriad of algal metabolites, only a few have been investigated (Asimakis et al., 2022). In continuation of these efforts, this study was designed to explore the potential use of L. papillosa and D. fasciola extracts and their synthesized nano-forms in the biological control of plant-parasitic nematodes. Based on the laboratory results, both marine algal extracts and their nano-forms have promising nematicidal activity and may be utilized in the production of new products that protect crops against plant-parasitic nematodes. The methanolic extract of D. fasciola was more effective in suppressing M. incognita egg hatching and eliminating its J2 as compared to water extract of L. papillosa. These outcomes align with Khan et al. (2015), who evaluated the antinemic potential of water and methanolic extracts of 32 seaweeds against M. javanica egg hatching and larval mortality.
The nematicidal activity of marine algal extracts was generally attributed to their content of secondary metabolites such as phenols, terpenes, alginate, sulfated galactans and carrageen (Asimakis et al., 2022; Khan & Mohammad, 2011; Shukla et al., 2016). Consequently, the nematicidal activity of D. fasciola could be attributed to the content of phenolic and flavonoid compounds, which play a role in nematicidal action; this finding agrees with Faizi et al. (2011), who identified that phytochemicals such as phenolic compounds are lethal to plant nematodes. They found that the isolated compounds, such as terthienyl, gallic, and linoleic acids, showed 100% mortality at the concentration of 0.125% for 24 h. Furthermore, Bano et al. (2020) found that the nematicidal ability of flavone and flavonol classes differed significantly depending on their structure, concentration, and exposure period. Kaempferol, myricetin, quercetin, and rutin have been shown to be fatal to the M. incognita J2. (Caboni et al., 2013; Faizi et al., 2011).
On the other hand, polysaccharides extracted from L. papillosa affected plant nematodes under laboratory conditions. Chemically, polysaccharides of L papillosa were characterized by Murad et al. (2017) as kappa, iota and lambda carrageenans (sulfated polysaccharides). The sulfate content of carrageenan types was found to be 20, 33 and 41%, respectively (Shukla et al., 2016). It is well documented that carrageenans act as elicitor molecules that enhance plant immunity against several pathogens (Stadnik & De Freitas, 2014). This activity could be due to the sulfate content (Sangha et al., 2015) or to its ability to inhibit the binding or entrance of pests and pathogens into the host cells (Ahmadi et al., 2015). Besides, the nematicidal activity of L. papillosa polysaccharides may be attributed to the osmotic pressure between the nematode body and the surrounding medium containing a high concentration of polysaccharides.
Under greenhouse conditions, it was noteworthy that algal extracts improved the vegetative growth parameters of tomato plants compared to the untreated control and Vydate treatments. These findings are in accordance by those of Ponnan et al. (2017) who stated that seaweed extracts could be used as pesticide and fertilizer. Furthermore, Shukla et al. (2016) revealed that seaweeds are a common source of plant growth regulators that possess auxin and cytokinin properties. Also, the prior treatments with D. fasciola and L. papillosa extracts considerably increased the phenolic content and the activities of polyphenol oxidase (PPO) and chitinase. Generally, marine algae promote the enzymatic and non-enzymatic antioxidant systems of the plants, including peroxidase, polyphenol oxidase, chitinase, -1, 3-glucanase, and phenolics, which are crucial for plant defense against infections (Ibrahim et al., 2021). The introduction of defense-related components and enhanced plant production served as additional indicators of plant health in addition to disease management (Baxter et al., 2014; Das & Roychoudhury, 2014).
The high production of these enzymes is thought to be one of the mechanisms by which the plant gains resistance to the plant harmful organisms and these results support those of Arioli et al. (2015). Chitinase and 1, 3-glucans can break down the cell wall of pests and release substances that promote the production of phytoalexins early and phenolic compounds as inductors of resistance (Silva et al., 2004). Chitinase frequently acts as a signal molecule to promote the synthesis of extra pathogenesis-related (PR) proteins or metabolites involved in plant defense mechanisms (Rahimi et al., 1998). Also, the secretion of polyphenol oxidase and peroxidase defense enzymes was assessed as potential improvements in the acquired systemic resistance (Bakr & Omar, 2018). Important biological processes such as the lignin production, degradation routes, and host defense systems all depend on these enzymes (Davies et al., 2008; El-Zawahry et al., 2021). Similarly, plants used phenolic compounds as part of their defense system against root-knot nematode infestations (Nikoo et al., 2014; Wuyts et al., 2006).
In comparison to Vydate and untreated control, our findings in Fig. 2 show that all treatments were quite successful in raising total sugar. This increase was attributed to plant resistance, where the nematode infestation reduced the sugar content in the infested plant. This finding agrees with Vaitheeswaran et al. (2011), who discovered that M. incognita infestation in plant tissue resulted in a lower amount of sugar, higher levels of proteins and lipids, and lower overall energy content. On the contrary, our result of increasing total sugar in all treatments compared with Vydate and untreated control disagrees with Gautam and Poddar (2014), who found a decrease in total sugar levels after nematode infestation, which reaches negligible sugar content after five weeks. According to our results, unlike the untreated control, all tested plant extracts increased total phenolic levels, in agreement with Abdel-Baset and Abdel-Monaim (2020) and Abdel-Monaim et al. (2017).
Generally, the algal extracts promoted self-defense and significantly improved the health status of tomato plants against nematode infections. This resulted in prolonging the fruiting life of tomato plants and in increasing the crop performance, fruit yield, fruit quality and post-harvest shelf-life. Furthermore, algal treatments reduced the nematode biological parameters (number of galls and egg masses) in tomato roots. Similar results were recorded in tomato, okra and sunflower treated with the aqueous and ethanolic extracts of Sargassum swartzii, S. tenerrimum, S. wightii, Melanothamnus afaqhusainii, Halimeda tuna and Spatoglossum variabile against Meloidogyne javanica (Sultana et al., 2012, 2018). They discovered that insecticides and seaweed extracts both had suppressive effects on tomato root infestation by preventing the J2 penetration into the roots and the formation of nematode galls.
On the other hand, the use of algal extract nano-forms in root-knot nematode control provides new trends that are safe, eco-friendly, and successful in combating Meloidogyne species. Nano-sized pesticide formulations might make it easier to effectively distribute the active ingredients to pests and pathogens and enhance plant nematode bio-control. Our results showed that the nano-forms were more effective on M. incognita galls and egg-masses compared to the original extracts of marine algae and untreated control; these results agree with Mohamed et al. (2021). This action attributed to nano-particles may have an inhibiting effect due to their physical makeup, which was essential for the nematode's ability to penetrate the cell wall (e.g., body form, size, and homogeneity) (Sharon et al., 2010). Moreover, both eukaryotic and prokaryotic cell responses to oxidative stress and ATP generation as well as membrane permeability are linked to this effect (Ahamed et al., 2010 and Lim et al., 2012). According to Nazir et al. (2019) and El-Habashy (2022), the nematicidal activity of pure extracts against the main phytoparasitic nematode, M. incognita, was significantly increased by their conversion to nanoparticles. Recently, marine algae have been considered dominant among all bio-sources, as they can produce excellent bioactive metabolites with potent biological activities, such as phenolic compounds, which are commonly found in plants and seaweeds (Zheng et al. 2020).
Conclusions
The present study uses nanotechnological techniques to improve the effectiveness of seaweed as a green, safe, and environmentally friendly method to control root-knot nematodes and to overcome and replace hazardous chemical nematicides. In addition to nematode control, these techniques increase plant health through the induction of defense-related enzymes, which are positively reflected in the vegetative plant growth and the yield and quality of tomato fruits. Due to their effectiveness against the root-knot nematodes and non-hazardous effects on the environment and to the enhancement of plant growth parameters, marine algal extracts (L. papillosa and D. fasciola) and their nano-forms are recommended as eco-friendly alternative solutions for controlling the root-knot nematode.
References
Abbott, W. S. (1925). A method of computing effectiveness of an insecticide. Journal of Economic Entomology, 18, 256–267. https://doi.org/10.1093/jee/18.2.265a
Abdel-Baset, S. H., & Abdel-Monaim, M. F. (2020). Nematicidal Potential of some Natural Botanical Extracts in Biocontrolling Meloidogyne javanica on Soybean under Laboratory and Greenhouse Conditions. Egyptian Journal of Agronematology, 19(1), 1–18. https://doi.org/10.21608/ejaj.2020.105859
Abd-Elgawad, M. M. M., Askary, T. H. (2015). Impact of phytonematodes on agriculture economy. In Askary T. H., Martinelli P. R. P. (Eds.) Biocontrol Agents of Phytonematodes, ( pp. 3–49). CABI: Wallingford, UK. https://doi.org/10.1079/9781780643755.0003
Abdelhafez, H. E. D. H., Abd Allah, A. A., Afify, M. M., Mahmoud, N. F., Guo, J., Murad, S. A., & Ibrahim, E. A. (2022). Protective action of polysaccharides from Laurencia papillose (Rhodophyta) against imidacloprid induced genotoxicity and oxidative stress in male albino rats. Environmental Analysis Health and Toxicology, 37(2), e2022011. https://doi.org/10.5620/eaht.2022011
Abdellatif, K. F., Abdelfattah, R. H., & El-Ansary, M. S. M. (2016). Green nanoparticles engineering on root-knot nematode infecting eggplants and their effect on plant DNA modification. Iranian Journal of Biotechnology, 14(4), 250–259. https://doi.org/10.15171/ijb.1309
Abdel-Monaim, M. F., Mazen, M., & Atwa, M. (2017). Effectiveness of plant extracts as safe control means against damping-off and root-rot diseases in faba bean plants. Egyptian Journal of Phytopathology, 45(1), 233–253. https://doi.org/10.21608/ejp.2017.89745
Ahamed, M., Posgai, R., Gorey, T. J., Nielsen, M., Hussain, S. M., & Rowe, J. J. (2010). Silver nanoparticles induced heat shock protein, oxidative stress and apoptosis in Drosophila melanogaster. Toxicology and Applied Pharmacology, 242, 263–269. https://doi.org/10.1016/j.taap.2009.10.016
Ahmadi, A., Moghadamtousi, S. Z., Abubakar, S., & Zandi, K. (2015). Antiviral potential of algae polysaccharides isolated from marine sources: A review. BioMed Research International, 41, 42. https://doi.org/10.1155/2015/825203
AOAC. (2012). Association of official analytical chemists. Official methods of analysis of the AOAC international no. 994.12. 19th edn, revision. 19th Chapter 4, 18–19, Official Journal of the European Communities 19.9.98, Gaithersburg, Maryland, USA.
Ara, J., Sultana, V., Ehtechamul Haque, S., Athar, M., & Qasim, R. (2002). Antibacterial activity of marine macroalgae from Karachi coast. The Bulletin of the Polish Academy of Sciences, 50, 199–206.
Araujo, J. C., Silva, P. P., Telhado, S. F., Sakai, R. H., Spoto, M. H., & Melo, P. C. (2014). Physico-chemical and sensory parameters of tomato cultivars grown in organic systems. Horticultura Brasileira, 32, 205–209. https://doi.org/10.1590/S0102-05362014000200015
Archidona-Yuste, A., Cantalapiedra-Navarrete, C., Lie’banas, G., Rapoport, H. F., Castillo, P., & Palomares-Rius, J. E. (2018). Diversity of root-knot nematodes of the genus Meloidogyne Goeldi. 1892. (Nematoda: Meloidogynidae) associated with olive plants and environmental cues regarding their distribution in southern Spain. PLoS One, 13(6), e0198236. https://doi.org/10.1371/journal.pone.0198236
Arioli, T., Mattner, S. W., & Winberg, P. C. (2015). Applications of seaweed extracts in Australian agriculture: Past, present and future. Journal of Applied Phycology, 27(5), 2007–2015. https://doi.org/10.1007/s10811-015-0574-9
Asimakis, E., Shehata, A. A., Eisenreich, W., Acheuk, F., Lasram, S., Asiouni, S., Emekci, M., Ntougias, S., Taner, G., May-Simera, H., Yilmaz, M., & Tsiamis, G. (2022). Algae and Their Metabolites as Potential Bio-Pesticides. Microorganisms, 10, 307. https://doi.org/10.3390/microorganisms10020307
Bakr, R. A., & Omar, A. H. (2018). Monitoring of systemic resistance induction in tomato against Meloidogyne incognita. J Plant Pathol Microbiol, 9(11), 2157–7471. https://doi.org/10.4172/2157-7471.1000464
Bano, S., Iqbal, E. Y., Zik-ur-Rehman, S., Fayyaz, S. N. D., & Faizi, S. H. (2020). Nematicidal activity of flavonoids with structure activity relationship (SAR) studies against root-knot nematodes Meloidogyne incognita. European Journal of Plant Pathology, 157, 299–309.
Barker, T. R. (1985). Nematode extraction and bioassays. In T. R. Barker, C. C. Carter, & J. N. Sasser (Eds.), An Advanced Treatise on Meloidogyne (Vol. II, pp. 19–35). North Carolina University.
Baxter, A., Mittler, R., & Suzuki, N. (2014). ROS as key players in plant stress signalling. Journal of Experimental Botany, 65(5), 1229–1240. https://doi.org/10.1093/jxb/ert375
Beerh, O. P., & Siddappa, G. S. (1959). A rapid spectrophotometric method for the detection and estimation of adulterants in tomato ketchup. Food Technology, 13, 414–418.
Benelli, G., Pavela, R., Maggi, F., Petrelli, R., & Nicoletti, M. (2017). Commentary: Making green pesticides greener? The potential of plant products for nanosynthesis and pest control. Journal of Cluster Science, 28, 3–10. https://doi.org/10.1007/s10876-016-1131-7
Ben-Hammouda, M., Kremer, R. J., Minor, H. C., & Sarwar, M. A. (1995). Chemical basis for differential allelopathic potential of sorghum hybrids on wheat. Journal of Chemical Ecology, 21, 775–786. https://doi.org/10.1007/BF02033460
Blunden, G. (1991). Agricultural uses of seaweeds and seaweed products. In M. D. Guiry (Ed.), Seaweed Resources in Europe: Uses and Potential (pp. 65–81). John Wiley and Sons.
Caboni, P., Saba, M., Tocco, G., Casu, L., Murgia, A., Maxia, A., Menkissoglu-Spiroudi, U., & Ntalli, N. (2013). Nematicidal activity of mint aqueous extracts against the root-knot nematode Meloidogyne incognita. Journal of Agriculture and Food Chemistry, 61(41), 9784–9788. https://doi.org/10.1021/jf403684h
Chen J.-X., Song B.-A. (2021). Natural nematicidal active compounds: Recent research progress and outlook. J Integr Agric, 20(8), 2015–2031. https://doi.org/10.1016/S2095-3119(21)63617-1
Craigie, J. S. (2011). Seaweed extracts stimuli in plant science and agriculture. J App Phyco, 23, 371–393. https://doi.org/10.1007/s10811-010-9560-4
Das, K., & Roychoudhury, A. (2014). Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Frontiers in Environmental Science, 2, 5310. https://doi.org/10.3389/fenvs.2014.00053
Davies, M. J., Hawkins, C. L., Pattison, D. I., & Rees, M. D. (2008). Mammalian heme peroxidases: From molecular mechanisms to health implications. Antioxid Redox, 10, 1199–1234. https://doi.org/10.1089/ars.2007.1927
Dubios, M., Gills, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28, 350–356. https://doi.org/10.1021/ac60111a017
Duncan, D. B. (1955). Multiple ranges and multiple F Test. Biometrics, 11, 11–24. https://doi.org/10.2307/3001478
El-Ansary, M. S. M., & Hamouda, R. A. (2014). Bio-control of root knot nematode infected banana plants by some marine algae. Russian Journal of Marine Biology, 40(2), 140–146. https://doi.org/10.1134/S1063074014020047
El Wakeil, N., Alkahtani, S., Gaafar, N. (2017). Is nanotechnology a promising field for insect pest control in IPM programs? New pesticides and soil sensors (pp. 273–329). Academic Press. https://doi.org/10.1016/B978-0-12-804299-1.00008-4
El-Habashy, D. E. (2022). Effectiveness of nanoparticles of some plant extracts against root-knot nematode, Meloidogyne incognita on tomato plants. SVU-International Journal of Agricultural Sciences, 4(3), 46–57. https://doi.org/10.21608/svuijas.2022.143524.1213
El-Nagdi, W. M. A., Abd-El-Khair, H., Soliman, G. M., & El-Sayed, G. M. (2019). Application of protoplast fusants of Bacillus licheniformis and Pseudomonas aeruginosa on Meloidogyne incognita in tomato and eggplant. Middle East Journal of Applied Sciences, 9, 622–629.
El-Zawahry, A., El Aref, H. M., Riad, S. N., & Zawam, H. S. (2021). Induction of Systemic Resistance in Tomato by some Abiotic Compounds Against Meloidogyne javanica. Assiut Journal of Agricultural Sciences, 52(1), 74–89. https://doi.org/10.21608/ajas.2021.171570
Faizi, S., Fayyaz, S., Bano, S., Iqbal, E. Y., Lubna Siddiqi, H., & Naz, A. (2011). Isolation of nematicidal compounds from Tagetespatula L. yellow flowers: Structure-activity relationship studies against cyst nematode Heteroderazea infective stage larvae. Journal of Agricultural and Food Chemistry, 59(17), 9080–9093. https://doi.org/10.1021/jf201611b
Fenical, W. (1982). Natural products chemistry in the marine environment. Science, 215, 923–928. https://doi.org/10.1126/science.215.4535.923
Fish, W. W., Perkins-Veazie, P., & Collins, J. K. (2002). A quantitative assay for lycopene that utilizes reduced volumes of organic solvents. Journal of Food Composition and Analysis, 15, 309–317. https://doi.org/10.1006/jfca.2002.1069
Gautam, S. K., & Poddar, A. N. (2014). Study on protein and sugar content in Meloidogyne incognita infected roots of bitter gourd. International Journal of Current Microbiology and Applied Sciences, 5, 470–478.
Hamouda, R. A., & El-Ansary, M. S. M. (2017). Potential of plant-parasitic nematode control in banana plants by microalgae as a new approach towards resistance. Egyptian Journal of Biological Pest Control, 27(2), 165–172.
Hussey, R. S., & Barker, K. R. (1973). A comparison of methods of collecting inocula of Meloidogyne spp. including a new technique. Plant Dis Report, 57, 1025–1028.
Ibañez, E., & Cifuentes, A. (2013). Benefits of Using Algae as Natural Sources of Functional Ingredients. Journal of the Science of Food and Agriculture, 93, 703–709. https://doi.org/10.1002/jsfa.6023
Ibrahim, D. S., Metwaly, H. A., & El-Sagheer, A. M. (2021). Synergistic Effect of Bioagents and Antioxidants against Root-Knot Nematode, Meloidogyne incognita on Sunflower. Egyptian Journal of Agronematology, 20(2), 140–158. https://doi.org/10.21608/ejaj.2021.187532
Ji-xiang, C., & Bao-an, S. (2021). Natural nematicidal active compounds: Recent research progress and outlook. Journal of Integrative Agriculture, 20(8), 2015–2031. https://doi.org/10.1016/S2095-3119(21)63617-1
Kayani, M. Z., Mukhtar, T., & Hussain, M. A. (2018). Interaction between nematode inoculum density and plant age on growth and yield of cucumber and reproduction of Meloidogyne incognita. Pakistan Journal of Zoology, 50, 897–902. https://doi.org/10.17582/journal.pjz/2018.50.3.897.902
Khalil, M. S., Abd El-Aziz, M. H., & Selim, R. E. S. (2022). Physiological and morphological response of tomato plants to nano-chitosan used against bio-stress induced by root-knot nematode (Meloidogyne incognita) and Tobacco mosaic tobamovirus (TMV). European Journal of Plant Pathology, 163, 799–812. https://doi.org/10.1007/s10658-022-02516-8
Khan, M. M., & Mohammad, T. A. (2011). Role of secondary metabolites in defense mechanisms of plants. Biol and Medicine, 3(2), 232–249.
Khan, S. A., Abid, M., & Hussain, F. (2015). Nematicidal activity of seaweeds against Meloidogyne javanica. Pakistan Journal of Nematology, 33(2), 195–203.
Lim, D., Roh, J. Y., Eom, H. J., Hyun, J. W., & Choi, J. (2012). Oxidative stress-related PMK-1 P38 MAPK activation as a mechanism for toxicity of silver nanoparticles to reproduction in the nematode Caenorhabditis elegans. Environ Toxicol Chemist, 31(3), 585–592. https://doi.org/10.1002/etc.1706
McCord, J. M., & Fridovich, I. (1969). Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). Journal of Biological Chemistry, 244(22), 6049–6055. https://doi.org/10.1016/S0021-9258(18)63504-5
Moens, M., Perry, R. N., & Starr, J. L. (2009). Meloidogyne species-a diverse group of novel and important plant parasites (pp. 1–17). In Root-knot nematodes Wallingford UK: CABI. https://doi.org/10.1079/9781845934927.0001
Mohamed, Y. M. A., Osman, S. A., Elshahawy, I. E., Soliman, G. M., & Ahmed, A. M. A. (2021). Charcoal rot and root-knot nematode control on faba bean by photosynthesized colloidal silver nanoparticles using bioactive compounds from Moringa oleifera leaf extract. Journal of Plant Protection Researchs, 61(4), 414–429. https://doi.org/10.24425/jppr.2021.139248
Mohammad, A. A., Amer, H. M., El-Sawy, S. M., Youssef, D. A., Nour, S. A., & Soliman, G. M. (2022). Nematicidal activity of sweet annie and garden cress nano-formulations and their impact on the vegetative growth and fruit quality of tomato plants. Science and Reports, 12(1), 22302. https://doi.org/10.1038/s41598-022-26819-2
Mukhtar, T., Arshad, I., Kayani, M. Z., Hussain, M. A., Kayani, S. B., Rahoo, A. M., & Ashfaq, M. (2013). Estimation of damage to okra (Abelmoschus esculentus) by root-knot disease incited by Meloidogyne incognita. Pak J of Bot, 45, 1023–1027.
Murad, H., Ghannam, A., Jazzara, M., Odeh, A., & Allaf, A. (2017). Isolation, structural characterization, and antiproliferative activity of phycocolloids from the red seaweed Laurencia papillosa on MCF-7 human breast cancer cells. International Journal of Biological Macromolecules, 108, 916–926. https://doi.org/10.1016/j.ijbiomac.2017.11.001
Nazir, K., Mukhtar, T., & Javed, H. (2019). In vitro effectiveness of silver nanoparticles against root-knot nematode (Meloidogyne incognita). Pakistan Journal of Zoology, 51(6), 2077–2083. https://doi.org/10.17582/journal.pjz/2019.51.6.2077.2083
Nikoo, F. S., Sahebani, N., Aminian, H., Mokhtarnejad, L., & Ghaderi, R. (2014). Induction of systemic resistance and defense-related enzymes in tomato plants using Pseudomonas fluorescens CHAO and salicylic acid against root-knot nematode Meloidogyne javanica. Journal of Plant Protection Research, 54(4), 383–389. https://doi.org/10.2478/jppr-2014-0057
Niu, B., Wang, W., Yuan, Z., Sederoff, R. R., Sederoff, H., Chiang, V. L., & Borriss, R. (2020). Microbial interactions within multiplestrain biological control agents impact soil borne plant disease. Frontiers in Microbiology, 11, 585404. https://doi.org/10.3389/fmicb.2020.585404
Ohri, P., & Pannu, S. K. (2010). Effect of phenolic compounds on nematodes- A review. Journal of Applied and Natural Science, 2(2), 344–350. https://doi.org/10.31018/jans.v2i2.144
Ojaghian, M. R., Wang, L., Qi Cui, Z., Yang, C., Zhongyun, T., & Xie, G. L. (2014). Anti-fungal and SAR potential of crude extracts derived from neem and ginger against storage carrot rot caused by Sclerotinia sclerotiorum. Industrial Crops and Products, 55, 130–139. https://doi.org/10.1016/j.indcrop.2014.02.012
Ong, S. N., Taheri, S., Othman, R. Y., & Teo, C. H. (2020). Viral disease of tomato crops (Solanumlycopesicum L.): An overview. Journal of Plant Diseases and Protection, 127, 725–739. https://doi.org/10.1007/s41348-020-00330-0
Ponnan, A., Ramu, K., Marudhamuthu, M., Marimuthu, R., Siva, K., & Kadarkarai, M. (2017). Antibacterial, antioxidant, and anticancer properties of Turbinaria conoides (J Agardh) Kuetz. Clinical Phytoscience, 3(5), 1–10.
Prasad, R., Bhattacharyya, A., & Nguyen, Q. D. (2017a). Nanotechnology in sustainable agriculture: Recent developments, challenges, and perspectives. Frontiers in Microbiology, 8, 1014. https://doi.org/10.3389/fmicb.2017.01014
Prasad, R., Gupta, N., Kumar, M., Kumar, V., Wang, S., & Abd-Elsalam, K. A. (2017). Nanomaterials act as plant defense mechanism. In J. Sangeetha & R. Prasad (Eds.), Nanotechnology: Food and Environmental Thangadurai D (pp. 253–269). Paradigm: Springer. https://doi.org/10.1007/978-981-10-4678-0_14
Rahimi, S., Wright, D. J., & Perry, R. N. (1998). Identification and localization of chitinases induced in the roots of potato plants infected with the potato cyst nematode Globodera pallida. Fundamental and Applied Nematology, 21(6), 705–713.
Rajesh, M., Prabhsharan, S., & Krishan, B. (1985). Nematicidal activity of some phenolic compounds against Meloidogyne incognita. Revue Nématol, 8(2), 161–164.
Rustiguel, C. B. (2012). Optimization of the chitinase production by different Metarhizium anisopliae strains under solid-state fermentation with silkworm chrysalis as substrate using CCRD. Advances in Microbiology, 2(03), 268. https://doi.org/10.4236/aim.2012.23032
Saad, M. G., Ghareeb, R. Y., & Saeed, A. A. (2019). The potential of endophytic fungi as biocontrol agents against the cotton leaf worm, Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae). Egyptian Journal of Biological Pest Control, 29(1), 1–7. https://doi.org/10.1186/s41938-019-0108-x
Saikia, S., Mahnot, N. K., & Mahanta, C. L. (2016). Phytochemical content and antioxidant activities of thirteen fruits of Assam. India Food Bioscience, 13, 15–20. https://doi.org/10.1016/j.fbio.2015.11.003
Sangha, J. S., Kandasamy, S., Khan, W., Bahia, N. S., Singh, R. P., & Critchley, A. T. (2015). λ-arrageenan suppresses Tomato Chlorotic Dwarf Viroid (TCDVd) replication and symptom expression in tomatoes. Mar Drugs, 13, 2875–2889. https://doi.org/10.3390/md13052875
Sharma, S. B., Mohiuddin, M., Jain, K. C., & Remanandan, P. (1994). Reaction of pigeonpea cultivars and germplasm accessions to the root-knot nematode, Meloidogyne javanica. Journal of Nematology, 26, 644–652.
Sharon, M., Choudhary, A. K., & Kumar, R. (2010). Nanotechnology in agricultural diseases and food safety. Journal of Phytological, 2(4), 83–92.
Shukla, P. S., Borza, T., Critchley, A. T., & Prithiviraj, B. (2016). Carrageenans from red seaweeds as promoters of growth and elicitors of defense response in plants. Frontiers in Marine Science, 3, 81. https://doi.org/10.3389/fmars.2016.00081
Silva, H. S. A., Romeiro, R. S., Macagnan, D., Halfeld-Vieira, B. A., Pereira, M. C. B., & Mounteer, A. (2004). Rhizobacterial induction of systemic resistance in tomato plants: Nonspecific protection and increase enzyme activities. Biological Control, 29(2), 288–295. https://doi.org/10.1016/S1049-9644(03)00163-4
Singh, H., Sharma, A., Bhardwaj, S. K., Arya, S. K., Bhardwaj, N., & Khatri, M. (2021). Recent advances in the applications of nano-agrochemicals for sustainable agricultural development. Environmental Sciences, 23(2), 213–239. https://doi.org/10.1039/D0EM00404A
Stadnik, M. J., & De Freitas, M. B. (2014). Algal polysaccharides as source of plant resistance inducers. Trop Plant Pathol, 39, 111–118. https://doi.org/10.1590/S1982-56762014000200001
Sultana, V., Baloc, G. N., Ara, J., Ehteshamul-Haque, S., Tariq, R. M., & Athar, M. (2012). Seaweeds as an alternative to chemical pesticides for the management of root diseases of sunflower and tomato. Journal of Applied Botany and Food Quality, 84, 162.
Sultana, V., Tariq, S., Hira, K., Tariq, A., Ara, J., Tariq, R. M., & Ehteshamul-Haque, S. (2018). Seaweed bio-fertilizer for the management of root rotting fungi and root knot nematodes affecting cotton crop. Pakistan Journal of Botany, 50(6), 2409–2412.
Vaitheeswaran, M., Ibrahim, S. M., & Lakshmi, D. (2011). Effect of Vitex negundo on some physiological aspects in root-knot nematode Meloidogyne incognita infected Hibiscus cannabinus. Medicinal Plants - International Journal of Phytomedicines and Related Industries, 3(3), 227–231. https://doi.org/10.5958/j.0975-4261.3.3.036
Vamos-Vigyazo, L., & Nadudvari-Markus, V. (1982). Enzymatic browning, polyphenol oxidase and peroxidase in pear cultivars. Acta Alimen, 11, 157–164.
Wang, L., Wang, X., Wu, H., & Liu, R. (2014). Overview on Biological Activities and Molecular Characteristics of Sulfated Polysaccharides from Marine Green Algae in Recent Years. Marine Drugs, 12, 4984–5020. https://doi.org/10.3390/md12094984
Worrall, E. A., Hamid, A., Mody, K. T., Mitter, N., & Pappu, H. R. (2018). Nanotechnology for Plant Disease Management. Agron, 12, 285. https://doi.org/10.3390/agronomy8120285
Wu, Y., Jenkins, T., Blunden, G., Whapham, C. A., & Hankins, S. D. (1997). The role of betaines in alkaline extracts of Ascophyllum nodosum in the reduction of Meloidogyne javanica and M. incognita infestations of tomato. Fundamental and Applied Nematology, 20(2), 99–102.
Wuyts, N., De Waele, D., & Swennen, R. (2006). Extraction and partial characterization of polyphenol oxidase from banana (Musa acuminate grande-naine) roots. Plant Physiology and Biochemistry, 44(4–5), 308–314. https://doi.org/10.1016/j.plaphy.2006.06.005
Yang, G., Zhou, B., Zhang, X., Zhang, Z., Wu, Y., Zhang, Y., et al. (2016). Effects of tomato root exudates on Meloidogyne incognita. PLoS ONE, 11, e0154675. https://doi.org/10.1371/journal.pone.0154675
Youssef, D. A. A., & Abdelmegeed, S. M. (2021). Polymer based encapsulation of peppermint oil (Menthapipreta) nanoemulsion and its effects on life and some physiological activities of honey bees Apis mellifera (Hymenoptera: Apidae). Egyptian Pharmaceutical Journal, 20(4), 313–322.
Zhang, Y., Zhu, S., Yin, L., Qian, F., Tang, C., & Yin, C. (2008). Preparation, characterization and biocompatibility of poly (ethylene glycol)-poly (n-butyl cyanoacrylate) nanocapsules with oil core via miniemulsion polymerization. European Polymer Journal, 44(6), 1654–1661. https://doi.org/10.1016/j.eurpolymj.2008.03.019
Zheng, L. X., Chen, X. Q., & Cheong, K. L. (2020). Current trends in marine algae polysaccharides: The digestive tract, microbial catabolism, and prebiotic potential. International Journal of Biological Macromolecules, 151, 344–354. https://doi.org/10.1016/j.ijbiomac.2020.02.168
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
G.S., S.N., A.M., S.E., S.M., D.Y., W.E., and E.I. conceived and planned the experiments. G.S., A.M., S.E., S.M., and E.I carried out the experiments. G.S., A.M., S.E., and E.I. contributed to sample preparation. G.S., S.N., A.M., S.E., S.M., D.Y., W.E., and E.I. contributed to the interpretation of the results. G.S. took the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis and manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Soliman, G.M., Nour, S.A., Mohammad, A.A. et al. Anti-nemic potential of Laurencia papillosa and Dilophys fasciola biosynthesized nano-extracts against tomato root-knot nematode Meloidogyne incognita. Phytoparasitica 52, 37 (2024). https://doi.org/10.1007/s12600-024-01157-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12600-024-01157-3