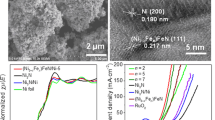
Abstract
Heterogeneous interfaces produced by inter-domain interactions on a nanoscale performs a crucial role in boosting the properties of an electrocatalyst toward oxygen evolution reaction (OER) process. Herein, a series of dual-phase electrodes with intimately connected heterointerfaces are prepared by in situ decomposing solid solution oxide of NixCoyFe100−x−yO, which grew on Ni foam massively via an ultrafast combustion approach. Particularly, with high-reaction kinetics caused by the reduction treatment at 450 °C, the less electronegative Fe and Co are more oxyphilic than Ni, which facilitated their co-exsolution and formation of CoFe2O4/NiO oxide with enriched oxygen vacancies. Benefiting from the nanoporous framework, heterojunction structure, and oxygen defects, the self-supporting electrodes present rapid charge/mass transmission and provide abundant active sites for OER. The optimized sample (R-SNCF4.5) shows low overpotentials of 226 and 324 mV at 10 and 100 mA·cm−2, a small Tafel slope (46.7 mV·dec−1), and excellent stability. The assembled R-SNCF4.5//Pt/C/NF electrolyzer demonstrates continuous electrolysis over 50 h at a current density of 10 mA·cm−2, under 1.51 V. Density functional theory (DFT) calculations verify that the strong electronic modulation plays a critical part in the CoFe2O4/NiO hybrid by lowering the energy barriers for the rate-determining steps, and Fe sites are the most active OER sites.
Graphical abstract

摘要
由纳米尺度上结构域间相互作用产生的异质界面在提高电催化剂的析氧反应 (OER) 性能方面发挥了关键作用。本文通过原位分解NixCoyFe100-x-yO固溶体氧化物制备了一系列具有紧密连接异质界面的双相电极。其中NixCoyFe100-x-yO固溶体氧化物通过超快燃烧法在泡沫镍表面原位、大规模生长制备。尤其在450oC的还原处理条件下会引起快速反应动力学, 因此电负性较低的Fe和Co亲氧性更高, 促进了二者的共同溶出并形成具有富集氧空位的CoFe2O4/NiO双相电极。得益于纳米多孔框架、异质结结构和氧缺陷, 该系列自支撑电极在OER过程中呈现出快速的电荷/质量传输, 并提供了丰富的活性位点。优化后的样品 (R-SNCF4.5) 在10和100 mA·cm−2的电流密度下分别显示出226和324 mV的低过电位, 同时具有小的Tafel斜率 (46.7 mV· dec−1) 和优异的稳定性。组装的R-SNCF4.5//Pt/C/NF全解水体系在1.51 V电位下, 10 mA·cm−2的电流密度下可连续电解50小时不发生电压增加。密度泛函理论计算证实, 有效的电子调控可以降低OER过程中决速步能垒, 并在CoFe2O4/NiO异质电极中起关键作用。该密度泛函理论计算结果也验证了Fe位点是最活跃的OER催化活性位点。








Similar content being viewed by others
References
Yuan F, Zhang D, Li ZJ, Sun HL, Yu QY,Wang QJ, Zhang JG, Wang B ,Wu YS., Xi K. Wang B. Unraveling the intercorrelation between micro/mesopores and K migration behavior in hard carbon. Small. 2022;18(12):2107113. https://doi.org/10.1002/smll.202107113.
Liu C, Li F, Ma LP, Cheng HM. Advanced materials for energy storage. Adv Mater. 2010;22:E28. https://doi.org/10.1002/adma.200903328.
Gong HY, Liang X, Sun GL, Li DW, Zheng XJ, Shi H, Zeng K, Xu GC, Li Y, Yang RZ, Yuan CZ. Insight into role of Ni/Fe existing forms in reversible oxygen catalysis based on Ni-Fe single-atom/nanoparticles and N-doped carbon. Rare Metals. 2022;12(41):4034. https://doi.org/10.1007/s12598-022-02078-y
Yue M, Lambert H, Pahon E, Roche R, Jemei S, Hissel D. Hydrogen energy systems: a critical review of technologies, applications, trends and challenges. Renew Sustain Energy Rev. 2021;146:111180. https://doi.org/10.1016/j.rser.2021.111180.
Guo S, Li X, Li J, Wei B. Boosting photocatalytic hydrogen production from water by photothermally induced biphase systems. Nat Commun. 2021;12:1. https://doi.org/10.1038/s41467-021-21526-4.
Lubitz W, Tumas W. Hydrogen: an overview. Chem Rev. 2007;107:3900. https://doi.org/10.1021/cr050200z.
Anantharaj S, Kundu S. Do the evaluation parameters reflect intrinsic activity of electrocatalysts in electrochemical water splitting? ACS Energy Lett. 2019;4:1260. https://doi.org/10.1021/acsenergylett.9b00686.
Zhu CZ, Shi QR, Feng S, Du D, Lin YH. Single-atom catalysts for electrochemical water splitting. ACS Energy Lett. 2018;3:1713. https://doi.org/10.1021/acsenergylett.8b00640.
Ifkovits ZP, Evans JM, Meier MC, Papadantonakis KM, Lewis NS. Decoupled electrochemical water-splitting systems: a review and perspective. Energy Environ Sci. 2021;14:4740. https://doi.org/10.1039/D1EE01226F.
Zhu KY, Zhu XF, Yang WS. Application of in situ techniques for the characterization of NiFe-based oxygen evolution reaction (OER) electrocatalysts. Angew Chemie Int Ed. 2019;58:1252. https://doi.org/10.1002/anie.201802923.
Jamesh M-I, Harb M. Tuning the electronic structure of the earth-abundant electrocatalysts for oxygen evolution reaction (OER) to achieve efficient alkaline water splitting—a review. J Energy Chem. 2021;56:299. https://doi.org/10.1016/j.jechem.2020.08.001.
Stoerzinger KA, Rao RR, Wang XR, Hong WT, Rouleau CM, Shao-Horn Y. The role of Ru redox in pH-dependent oxygen evolution on rutile ruthenium dioxide surfaces. Chem. 2017;2:668. https://doi.org/10.1016/j.chempr.2017.04.001.
Reier T, Oezaslan M, Strasser P. Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: a comparative study of nanoparticles and bulk materials. Acs Catal. 2012;2:1765. https://doi.org/10.1021/cs3003098.
Cui H, Liao HX, Wang ZL, Xie JP, Tan PF, Chu DW, Jun P. Synergistic electronic interaction between ruthenium and nickel-iron hydroxide for enhanced oxygen evolution reaction. Rare Met. 2022;41:2606. https://doi.org/10.1007/s12598-022-02003-3.
Vishnu B, Mathi S, Sriram S, Jayabharathi J. Greenly reduced CoFe-PBA/Nickel foam: a robust dual electrocatalyst for solar-driven alkaline water electrolysis with a low cell voltage. ChemistrySelect. 2022;7:e202201682. https://doi.org/10.1002/slct.202201682.
Dong GF, Xie FY, Kou FX, Chen TT, Wang FY, Zhou YW, Wu KC, Du SW, Fang M, C. Ho J. NiFe-layered double hydroxide arrays for oxygen evolution reaction in fresh water and seawater. Mater Today Energy. 2021;22:100883. https://doi.org/10.1016/j.mtener.2021.100883
Yan Y, Liu HC, Liu CY, Zhao YG, Liu SZ, Wang D, Fritz M, Ispas A, Bund A, Schaaf P, Wang XY. Efficient preparation of Ni-M (M= Fe Co, Mo) bimetallic oxides layer on Ni nanorod arrays for electrocatalytic oxygen evolution. Appl Mater Today. 2021;25: 101185. https://doi.org/10.1016/j.apmt.2021.101185.
Nagappan S, Karmakar A, Madhu R, Selvasundarasekar SS, Kumaravel S, Bera K, Dhandapani HN, Sarkar D, Yusuf SM, Kundu S. 2D CoFe-LDH nanosheet-incorporated 1D microfibers as a high-performance OER electrocatalyst in neutral and alkaline media. ACS Appl Energy Mater. 2022;5(9):11483. https://doi.org/10.1021/acsaem.2c01964.
Zhang ZJ, Guo JP, Sun SH, Sun Q, Zhao YW, Zhang YF, Yu ZY, Li CS, Sun Y, Zhang MM, Jiang Y. Optimized valence state of Co and Ni in high-entropy alloy for high active-stable OER. Rare Met. 2023. https://doi.org/10.1007/s12598-023-02448-0.
Li GJ, Tang YB, Fu TT, Xiang Y, Xiong ZP, Si YJ, Guo CZ, Jiang ZQ. S, N co-doped carbon nanotubes coupled with CoFe nanoparticles as an efficient bifunctional ORR/OER electrocatalyst for rechargeable Zn-air batteries. Chem Eng J. 2022;429: 132174. https://doi.org/10.1016/j.cej.2021.132174.
Li X, Kou ZK, Xi SB, Zang WJ, Yang T, Zhang L, Wang J. Porous NiCo2S4/FeOOH nanowire arrays with rich sulfide/hydroxide interfaces enable high OER activity. Nano Energy. 2020;78:105230. https://doi.org/10.1016/j.nanoen.2020.105230.
Chen M, Hu YP, Liang K, Zhao ZM, Luo YT, Luo S, Ma JT. Interface engineering triggered by carbon nanotube-supported multiple sulfides for boosting oxygen evolution. Nanoscale. 2021;13:18763. https://doi.org/10.1039/D1NR04540G.
Singh S, Nguyen DC, Kim NH, Lee JH. Interface engineering induced electrocatalytic behavior in core-shelled CNTs@ NiP2/NbP heterostructure for highly efficient overall water splitting. Chem Eng J. 2022;442:136120. https://doi.org/10.1016/j.cej.2022.136120.
Galani SM, Mondal A, Srivastava DN, Panda AB. Development of RuO2/CeO2 heterostructure as an efficient OER electrocatalyst for alkaline water splitting. Int J Hydrogen Energy. 2020;45:18635. https://doi.org/10.1016/j.ijhydene.2019.08.026.
Zubair M, Hassan MMU, Mehran MT, Baig MM, Hussain S, Shahzad F. 2D MXenes and their heterostructures for HER, OER and overall water splitting: a review. Int J Hydrogen Energy. 2022;47:2794. https://doi.org/10.1016/j.ijhydene.2021.10.248.
Saravanakumar T, Sathiya Bama S, Selvaraju T, Sardhar Basha SJ. Hexacyanoferrate-complex-derived NiFe2O4/CoFe2O4 heterostructure–MWCNTs for an efficient oxygen evolution reaction. Energy Fuels. 2021;35:5372. https://doi.org/10.1021/acs.energyfuels.0c04224.
Fu ZQ, Liu SL, Mai ZQ, Tang ZH, Qin DD, Tian Y, Wang XF. Heterostructure and oxygen vacancies promote NiFe2O4/Ni3S4 toward oxygen evolution reaction and Zn-Air batteries. Chem Asian J. 2020;15:3568. https://doi.org/10.1002/asia.202001033.
Liu ZZ, Xiong SJ, Peng X. Oxygen evolution reaction property of cobalt vanadate nanosheets. Chin J Rare Met. 2022;46(6):839. https://doi.org/10.13373/j.cnki.cjrm.XY21070006
Balasubramanian P, He S-B, Jansirani A, Deng HH, Peng HP, Xia XH, Chen W. Engineering of oxygen vacancies regulated core-shell N-doped carbon@ NiFe2O4 nanospheres: a superior bifunctional electrocatalyst for boosting the kinetics of oxygen and hydrogen evaluation reactions. Chem Eng J. 2021;405: 126732. https://doi.org/10.1016/j.cej.2020.126732.
Asnavandi M, Yin YC, Li YB, Sun CH, Zhao C. Promoting oxygen evolution reactions through introduction of oxygen vacancies to benchmark NiFe–OOH catalysts. ACS Energy Lett. 2018;3:1515. https://doi.org/10.1021/acsenergylett.8b00696.
Reier T, Pawolek Z, Cherevko S, Bruns M, Jones T, Teschner D, Selve S, Bergmann A, Nong HA, Schlögl R, Mayrhofer KJJ, Strasser P. Molecular insight in structure and activity of highly efficient, low-Ir Ir–Ni oxide catalysts for electrochemical water splitting (OER). J Am Chem Soc. 2015;137:13031. https://doi.org/10.1021/jacs.5b07788.
Xue Z, Zhang XY, Qin JQ, Liu RP. Revealing Ni-based layered double hydroxides as high-efficiency electrocatalysts for the oxygen evolution reaction: a DFT study. J Mater Chem A. 2019;7:23091. https://doi.org/10.1039/C9TA06686A.
Shen JJ, Zheng XQ, Peng LS, Waterhouse GIN, Tan LQ, Yang J, Li L, Wei ZD. Heteroatom modification of nanoporous nickel surfaces for electrocatalytic water splitting. ACS Appl Nano Mater. 2020;3:11298. https://doi.org/10.1021/acsanm.0c02391.
Liu H, Xi C, Xin JH, Zhang GL, Zhang SF, Zhang ZJ, Huang Q, Li JX, Liu H, Kang JL. Free-standing nanoporous NiMnFeMo alloy: an efficient non-precious metal electrocatalyst for water splitting. Chem Eng J. 2021;404:126530. https://doi.org/10.1016/j.cej.2020.126530.
Ahmad I, Ahmed J, Batool S, Zafar MN, Hanif A, Zahidullah, Nazar MF, Ul-Hamid A, Jabeen U, Dahshan A, Idrees M, Shehzadi SA. Design and fabrication of Fe2O3/FeP heterostructure for oxygen evolution reaction electrocatalysis. J Alloys Compd. 2022;894:162409. https://doi.org/10.1016/j.jallcom.2021.162409
Xu H, Shang HY, Wang C, Jin LJ, Chen CY, Wang CY, Du YK. Three-dimensional open CoMoOx/CoMoSx/CoSx nanobox electrocatalysts for efficient oxygen evolution reaction. Appl Catal B Environ. 2020;265:118605. https://doi.org/10.1016/j.apcatb.2020.118605.
Aruchamy G, Thangavelu S. Bifunctional CoSn(OH)6/MnO2 composite for solid-state asymmetric high power density supercapacitor and for an enhanced OER. Electrochim Acta. 2020;344: 136141. https://doi.org/10.1016/j.electacta.2020.136141.
Zhao LX, Ge HY, Zhang GH, Wang FB, Li GD. Hierarchical Ni3S2-CoMoSx on the nickel foam as an advanced electrocatalyst for overall water splitting. Electrochim Acta. 2021;387: 138538. https://doi.org/10.1016/j.electacta.2021.138538.
Saad A, Liu DQ, Wu YC, Song ZQ, Li Y, Najam T, Zong K, Tsiakaras P, Cai XK. Ag nanoparticles modified crumpled borophene supported Co3O4 catalyst showing superior oxygen evolution reaction (OER) performance. Appl Catal B Environ. 2021;298:120529. https://doi.org/10.1016/j.apcatb.2021.120529.
Kim HJ, Kim HY, Joo J, Joo SH, Lim JS, Lee J, Huang HW, Shao MH, Hu J, Kim JY, Min BJ, Lee SW, Kang M, Lee K, Choi S, Park Y, Wang Y, Li JJ, Zhang ZC, Ma JM, Choi S-I. Recent advances in non-precious group metal-based catalysts for water electrolysis and beyond. J Mater Chem A. 2022;10:50. https://doi.org/10.1039/D1TA06548C.
Kim B, Kabiraz MK, Lee J, Choi C, Baik H, Jung Y, Oh SH, Choi SI, Lee K. Vertical-crystalline Fe-doped β-Ni oxyhydroxides for highly active and stable oxygen evolution reaction. Matter. 2021;4:3585. https://doi.org/10.1016/j.matt.2021.09.003.
Dionigi F, Strasser P. NiFe-based (oxy) hydroxide catalysts for oxygen evolution reaction in non-acidic electrolytes. Adv Energy Mater. 2016;6:1600621. https://doi.org/10.1002/aenm.201600621.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 52101251) and the Natural Science Foundation of Hebei Province (Nos. E2020208069 and B2020208083).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, SF., Yin, XL., Wang, J. et al. In situ regulating intimately connected heterostructure by decomposition of solid solution oxides toward high-efficient water oxidation. Rare Met. 43, 1557–1569 (2024). https://doi.org/10.1007/s12598-023-02536-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-023-02536-1