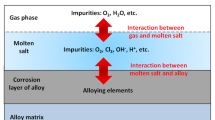
Abstract
Thermal energy storage (TES) systems based on molten salt are widely used in concentrating solar power (CSP) plants. The investigation of the corrosion behavior of alloy materials in molten salt is crucial for the correct selection of alloy materials and the design of TES systems. In this study, the corrosion behavior of 304, 310S, 316, and In625 alloys in molten chloride salts (27 mol% NaCl-22 mol% KCl-51 mol% MgCl2) was investigated. The evolution of mass loss of the alloy samples with corrosion time and temperature and the analysis of the experimental results by scanning electron microscopy (SEM), energy dispersive spectrometer (EDS), and X-ray diffraction (XRD) revealed the corrosion mechanism of the alloy samples in molten chloride salts. The main factors affecting the corrosion of the alloy samples were further analyzed. It was found that the loose multi-layer corrosion was formed on the surface of the corroded alloy samples with the increase in corrosion degree. Moreover, the experimental results showed that Mo played a positive role in improving the corrosion resistance of the alloy samples because the presence of Mo could inhibit the outward diffusion of alloying element Cr. This work enriches the molten salt corrosion database and provides a reference for the selection of alloy materials for TES systems with potential application in CSP plants.
Graphical abstract

摘要
基于熔盐的热能储存 (TES) 系统被广泛应用于聚光太阳能热发电。研究合金材料在熔盐中的腐蚀行为对于合金材料的正确选择和TES系统的设计至关重要。在这项研究中,评估了304、310S、316和In625 四种合金在熔融氯化物盐(27 mol% NaCl-22 mol% KCl-51 mol% MgCl2)中的腐蚀行为。研究了合金样品的质量损失随腐蚀时间和温度的演变规律。通过扫描电子显微镜、能谱仪和X射线衍射对实验结果的分析揭示了合金样品在熔融氯化物盐中的腐蚀机理。还进一步分析了影响合金试样腐蚀的主要因素。随着腐蚀程度的增加,被腐蚀的合金试样表面形成疏松的多层腐蚀层。此外,实验结果表明Mo对提高合金样品的耐蚀性起到了积极作用,Mo的存在可以抑制合金元素Cr的向外扩散。该工作丰富了熔盐腐蚀数据库,为光热发电厂TES系统合金材料的选择提供了参考。










Similar content being viewed by others
References
Zhang MH, Qi JL, Liu YQ, Lan S, Luo ZX, Pan H, Lin YH. High energy storage capability of perovskite relaxor ferroelectrics via hierarchical optimization. Rare Met. 2022;41(3):730. https://doi.org/10.1007/s12598-021-01869-z.
Qin YC, Wang FQ, Wang XM, Wang MW, Zhang WL, An WK, Wang XP, Ren YL, Zheng X, Lv DC, Ahmad A. Noble metal-based high-entropy alloys as advanced electrocatalysts for energy conversion. Rare Met. 2021;40(9):2354. https://doi.org/10.1007/s12598-021-01727-y.
Ke GL, Jia B, He HC, Zhou Y, Zhou M. State-of-the-art advancements of transition metal oxides as photoelectrode materials for solar water splitting. Rare Met. 2022;41(7):2370. https://doi.org/10.1007/s12598-022-01968-5.
Han DM, Lougou BG, Shuai Y, Wang W, Jiang BS, Shagdar E. Study of thermophysical properties of chloride salts doped with CuO nanoparticles for solar thermal energy storage. Sol Energy Mater Sol Cells. 2022;234:111432. https://doi.org/10.1016/j.solmat.2021.111432.
Fernández AG, Gomez-Vidal J, Oró E, Kruizenga A, Solé A, Cabeza LF. Mainstreaming commercial CSP systems: a technology review. Renew Energ. 2019;140:152. https://doi.org/10.1016/j.renene.2019.03.049.
Herrmann U, Kelly B, Price H. Two-tank molten salt storage for parabolic trough solar power plants. Energy. 2004;29:883. https://doi.org/10.1016/S0360-5442(03)00193-2.
Pacheco JE, Gilbert R. Overview of recent results for the solar two test and evaluations program. In: Proceedings of the ASME international solar energy conference 1999: renewable and advanced energy systems for the 21st century. Maui; 1999.1125.
Ren LZ, Zhao XG, Zhang YZ, Li YB. The economic performance of concentrated solar power industry in China. J Clean Prod. 2018;205:799. https://doi.org/10.1016/j.jclepro.2018.09.110.
Delise T, Tizzoni AC, Ferrara M, Corsaro N, D’Ottavi C, Sau S, Licoccia S. Thermophysical, environmental, and compatibility properties of nitrate and nitrite containing molten salts for medium temperature CSP applications: a critical review. J Eur Ceram Soc. 2019;39:92. https://doi.org/10.1016/j.jeurceramsoc.2018.07.057.
Arias I, Cardemil J, Zarza E, Valenzuela L, Escobar R. Latest developments, assessments and research trends for next generation of concentrated solar power plants using liquid heat transfer fluids. Renew Sust Energ Rev. 2022;168:112844. https://doi.org/10.1016/j.rser.2022.112844.
Ding WJ, Bauer T. Progress in research and development of molten chloride salt technology for next generation concentrated solar power plants. Engineering. 2021;7:334. https://doi.org/10.1016/j.eng.2020.06.027.
Sang LX, Cai M, Zhao YB, Ren N, Wu YT, Burda C. Mixed metal carbonates/hydroxides for concentrating solar power analyzed with DSC and XRD. Sol Energy Mater Sol Cells. 2015;140:167. https://doi.org/10.1016/j.solmat.2015.04.006.
Wu YT, Ren N, Wang T, Ma CF. Experimental study on optimized composition of mixed carbonate salt for sensible heat storage in solar thermal power plant. Sol Energy. 2011;85:1957. https://doi.org/10.1016/j.solener.2011.05.004.
Olivares RI, Chen CL, Wright S. The thermal stability of molten lithium-sodium-potassium carbonate and the influence of additives on the melting point. J Sol Energy Eng. 2012;134(4):041002. https://doi.org/10.1115/1.4006895.
Myers PD Jr, Goswami DY. Thermal energy storage using chloride salts and their eutectics. Appl Therm Eng. 2016;109:889. https://doi.org/10.1016/j.applthermaleng.2016.07.046.
D’Souza B, Leong A, Yang QF, Zhang JS. Corrosion behavior of boronized nickel-based alloys in the molten chloride Salt. Corros Sci. 2021;182:109285. https://doi.org/10.1016/j.corsci.2021.109285.
Bell S, Steinberg T, Will G. Corrosion mechanisms in molten salt thermal energy storage for concentrating solar power. Renew Sust Energ Rev. 2019;114:109328. https://doi.org/10.1016/j.rser.2019.109328.
Hu Z, Liu LL, Lu PF, Liu WH, Zhang F, Tang ZF. Corrosion behavior and mechanism of 316 stainless steel in NaCl-KCl-ZnCl2 molten salts at high temperature. Mater Today Commun. 2022;31:103297. https://doi.org/10.1016/j.mtcomm.2022.103297.
Vignarooban K, Pugazhendhi P, Tucker C, Gervasio D, Kannan AM. Corrosion resistance of Hastelloys in molten metal-chloride heat-transfer fluids for concentrating solar power applications. Sol Energy. 2014;103:62. https://doi.org/10.1016/j.solener.2014.02.002.
Sun H, Wang JQ, Li ZJ, Zhang P, Su XZ. Corrosion behavior of 316SS and Ni-based alloys in a ternary NaCl-KCl-MgCl2 molten salt. Sol Energy. 2018;171:320. https://doi.org/10.1016/j.solener.2018.06.094.
Liu X, Jia RL, Zhang HX, Guo WM, Bai Y, Ma W. Corrosion behavior and mechanical properties of FSW joint for 5083 aluminum alloy with different shaft shoulder diameters. Chin J Rare Met. 2022;46(8):1006. https://doi.org/10.13373/j.cnki.cjrm.XY21020010.
Han DM, Lougou BG, Xu YT, Shuai Y, Huang X. Thermal properties characterization of chloride salts/nanoparticles composite phase change material for high-temperature thermal energy storage. Appl Energy. 2020;264:114674. https://doi.org/10.1016/j.apenergy.2020.114674.
ACG-oCo Metals. Standard practice for preparing, cleaning, and evaluating corrosion test specimens. ASTM international. 2017. https://www.academia.edu/39029280/ASTM_G1_Standard_Practice_for_Preparing_Cleaning_and_Evaluation_Corrosion_Test_Specimens. Accessed 25 Jan 2023.
Lai X, Yin HQ, Li P, Liu BX, Gao L, Tang ZF. The corrosion behavior of 304 stainless steel in NaNO3-NaCl-NaF molten salt and vapor. RSC Adv. 2022;12:7157. https://doi.org/10.1039/D2RA00364C.
Liu B, Wei XL, Wang WL, Lu JF, Ding J. Corrosion behavior of Ni-based alloys in molten NaCl-CaCl2-MgCl2 eutectic salt for concentrating solar power. Sol Energy Mater Sol Cells. 2017;170:77. https://doi.org/10.1016/j.solmat.2017.05.050.
Liu Q, Xu HX, Yin HQ, Li N, Wang WR, Li LF, Tang ZF, Qian Y. Corrosion behaviour of 316 stainless steel in NaCl-KCl-MgCl2 salt vapour at 700 °C. Corros Sci. 2022;194:109921. https://doi.org/10.1016/j.corsci.2021.109921.
Grégoire B, Oskay C, Meißner TM, Galetz MC. Corrosion mechanisms of ferritic-martensitic P91 steel and Inconel 600 nickel-based alloy in molten chlorides. Part II: NaCl-KCl-MgCl2 ternary system. Sol Energy Mater Sol Cells. 2020;216:110675. https://doi.org/10.1016/j.solmat.2020.110675.
Gong Q, Shi H, Chai Y, Yu R, Weisenburger A, Wang DH, Bonk A, Bauer T, Ding WJ. Molten chloride salt technology for next-generation CSP plants: compatibility of Fe-based alloys with purified molten MgCl2-KCl-NaCl salt at 700 °C. Appl Energy. 2022;324:119708. https://doi.org/10.1016/j.apenergy.2022.119708.
Liu Q, Wang ZR, Liu WH, Yin HQ, Tang ZF, Qian Y. Ni-Mo-Cr alloy corrosion in molten NaCl-KCl-MgCl2 salt and vapour. Corros Sci. 2021;180:109183. https://doi.org/10.1016/j.corsci.2020.109183.
Galetz MC, Rammer B, Schütze M. Refractory metals and nickel in high temperature chlorine-containing environments-thermodynamic prediction of volatile corrosion products and surface reaction mechanisms: a review. Mater Corros. 2015;66:1206. https://doi.org/10.1002/maco.201408130.
Izzuddin H, Hayashi S, Yoneda S, Kogin T, Ishikawa E, Noguchi M. Effect of Mo on corrosion behavior of Ni20Cr-xMo alloys in air with NaCl-KCl-CaCl2 vapor at 570 °C. Mater Corros. 2020;71:1488. https://doi.org/10.1002/maco.201911469.
Ouyang FY, Chang CH, Kai JJ. Long-term corrosion behaviors of Hastelloy-N and Hastelloy-B3 in moisture-containing molten FLiNaK salt environments. J Nucl Mater. 2014;446(1–3):81. https://doi.org/10.1016/j.jnucmat.2013.11.045.
Zahs A, Spiegel M, Grabke HJ. Chloridation and oxidation of iron, chromium, nickel and their alloys in chloridizing and oxidizing atmospheres at 400–700 °C. Corros Sci. 2000;42(6):1093. https://doi.org/10.1016/S0010-938X(99)00142-0.
Bender R, Schütze M. The role of alloying elements in commercial alloys for corrosion resistance in oxidizing-chloridizing atmospheres. Part I: literature evaluation and thermodynamic calculations on phase stabilities. Mater Corros. 2003;54:567. https://doi.org/10.1002/maco.200390129.
Acknowledgements
This study was financially supported by the China National Key Research and Development Plan Project (No. 2018YFA0702300) and the National Natural Science Foundation of China (Nos. 52227813 and 51950410590).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, DM., Shuai, Y., Lougou, B.G. et al. Corrosion evaluation and resistance study of alloys in chloride salts for concentrating solar power plants. Rare Met. 43, 1222–1233 (2024). https://doi.org/10.1007/s12598-023-02506-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-023-02506-7