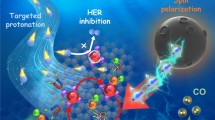
Abstract
Double-exchange (DE) interaction plays an important role in electrocatalytic oxygen evolution reaction (OER). However, precise achievement of DE interaction often requires foreign dopants or vacancy engineering, leading to destabilization of the catalysts and deterioration of performance. By contrast, the utilization of environmentally friendly, contactless, and continuously adjustable magnetic fields to study the OER process is profitable to avoid aforementioned interference factors and further elucidate the direct relationship 0.5between DE interaction and OER activity. Here, by using cobalt hydroxide carbonate (Co(OH)(CO3)·xH2O, CoHC) nanostructures as a proof-of-concept study, external magnetic fields are carefully implemented to verify the role of DE interaction during water oxidation reaction. Detailed studies reveal that external magnetic fields effectively enhance the reaction rate of the catalyst, the overpotential decreases from 386 to 355 mV (100 mA·cm−2), while Tafel slopes drastically decline from 93 to 67 mV·dec−1 (1.0 T). Moreover, magnetic field increment exhibits robust durability. Through in situ Raman and impedance measurements under external field, it can be found that magnetic field promotes the electron migration between Co2+ and Co3+ in the CoHC catalysts with the assistance of DE interactions, thus boosting the OER efficiency.
Graphical abstract

摘要
双交换相互作用在电催化氧化反应中扮演着重要作用。然而,精确实现双交换相互作用往往需通过掺杂或空位调控等方法,导致催化剂的不稳定以及性能衰退。相比之下,利用环境友好、无接触且连续可调的外磁场来研究电催化氧化过程,可以避免上述干扰因素,有助于进一步阐明双交换相互作用和氧化反应活性之间的关系。本文中,我们以碱式碳酸钴(Co(OH)(CO3)0.5·xH2O, CoHC)纳米结构为模型,施加外部磁场进而验证双交换机制在水氧化反应中的作用。研究结果显示,外部磁场有效地提高了催化剂的反应速度,在100 mA·cm-2电流密度下,过电位从386 mV降至355 mV,而塔菲尔斜率从93 mV·dec-1下降到67 mV·dec-1(1.0 T)。此外,磁场下的电化学性能表现出优异的耐久性。通过原位拉曼和阻抗谱测量,我们发现在双交换相互作用的帮助下,外磁场促进了CoHC催化剂中Co2+和Co3+之间的电子迁移,进而提高了电催化氧化的效率。





Similar content being viewed by others
References
Suen NT, Huang SF, Quan Q, Zhang N, Xu YJ, Chen HM. Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives. Chem Soc Rev. 2017;46(2):337. https://doi.org/10.1039/C6CS00328A.
Wei ZX, Zhu YT, Liu JY, Zhang ZC, Hu WP, Xu H, Fang YZ, Ma JM. Recent advance in single-atom catalysis. Rare Met. 2021;40(4):767. https://doi.org/10.1007/s12598-020-01592-1.
Sun JP, Zhao Z, Li J, Li ZZ, Meng XC. Recent advances in electrocatalytic seawater splitting. Rare Met. 2022;42(3):751. https://doi.org/10.1007/s12598-022-02168-x.
Cao SY, Ye F, Zhang NN, Guo YL, Guo Y, Wang L, Dai S, Zhan WC. Synergistic effect of bimetallic RuPt/TiO2 catalyst in methane combustion. Rare Met. 2022;42(1):165. https://doi.org/10.1007/s12598-022-02118-7.
Li L, Shi YX, Hou M, Zhang ZC. Research progress of copper-based materials for electrocatalytic CO2 reduction reaction. Chin J Rare Met. 2022;46(6):681. https://doi.org/10.13373/j.cnki.cjrm.XY21120017.
Song J, Wei C, Huang ZF, Liu C, Zeng L, Wang X, Xu ZJ. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem Soc Rev. 2020;49(7):2196. https://doi.org/10.1039/C9CS00607A.
Kim JS, Kim B, Kim H, Kang K. Recent progress on multimetal oxide catalysts for the oxygen evolution reaction. Adv Energy Mater. 2018;8(11):1702774. https://doi.org/10.1002/aenm.201702774.
Yang ZX, Li XG, Yao QL, Lu ZH, Zhang N, Xia J, Yang K, Wang YQ, Zhang K, Liu HZ, Zhang LT, Lin HJ, Zhou QJ, Wang F, Yu ZM, Ma JM. 2022 roadmap on hydrogen energy from production to utilizations. Rare Met. 2022;41(10):3251. https://doi.org/10.1007/s12598-022-02029-7.
Liu LH, Li N, Han M, Han JR, Liang HY. Scalable synthesis of nanoporous high entropy alloys for electrocatalytic oxygen evolution. Rare Met. 2022;41(1):125. https://doi.org/10.1007/s12598-021-01760-x.
Liu NZ, Xiong SJ, Peng X. Oxygen evolution reaction property of cobalt vanadate nanosheets. Chin J Rare Met. 2022;46(6):839. https://doi.org/10.13373/j.cnki.cjrm.XY21070006.
Wang L, Stoerzinger KA, Chang L, Zhao J, Li Y, Tang CS, Yin X, Bowden ME, Yang Z, Guo HZ, You L, Guo R, Wang J, Ibrahim K, Chen J, Rusydi A, Wang J, Chambers SA, Du Y. Tuning bifunctional oxygen electrocatalysts by changing the A-site rare-earth element in perovskite nickelates. Adv Funct Mater. 2018;28(39):1803712. https://doi.org/10.1002/adfm.201803712.
Song F, Bai L, Moysiadou A, Lee S, Hu C, Liardet L, Hu X. Transition metal oxides as electrocatalysts for the oxygen evolution reaction in alkaline solutions: an application-inspired renaissance. J Am Chem Soc. 2018;140(25):7748. https://doi.org/10.1021/jacs.8b04546.
Han L, Dong S, Wang E. Transition-metal (Co, Ni, and Fe)-based electrocatalysts for the water oxidation reaction. Adv Mater. 2016;28(42):9266. https://doi.org/10.1002/adma.201602270.
Biz C, Fianchini M, Gracia J. Strongly correlated electrons in catalysis: focus on quantum exchange. ACS Catal. 2021;11(22):14249. https://doi.org/10.1021/acscatal.1c03135.
Cao L, Luo Q, Chen J, Wang L, Lin Y, Wang H, Liu X, Shen X, Zhang W, Liu W, Qi Z, Jiang Z, Yang J, Yao T. Dynamic oxygen adsorption on single-atomic ruthenium catalyst with high performance for acidic oxygen evolution reaction. Nat Commun. 2019;10:4849. https://doi.org/10.1038/s41467-019-12886-z.
Ren X, Wu T, Sun Y, Li Y, Xian G, Liu X, Shen C, Gracia J, Gao HJ, Yang H, Xu ZJ. Spin-polarized oxygen evolution reaction under magnetic field. Nat Commun. 2021;12:2608. https://doi.org/10.1038/s41467-021-22865-y.
Craig MJ, García-Melchor M. Reaction descriptors for the oxygen evolution reaction: recent advances, challenges, and opportunities. Curr Opin Electrochem. 2022;35:101044. https://doi.org/10.1016/j.coelec.2022.101044.
Anderson PW, Hasegawa H. Considerations on double exchange. Phys Rev. 1955;100(2):675. https://doi.org/10.1103/PhysRev.100.675.
Li J, Che D, Dong H, Baker DR, Jiang R. Boosted oxygen evolution reactivity by igniting double exchange interaction in spinel oxides. J Am Chem Soc. 2020;142(1):50. https://doi.org/10.1021/jacs.9b10882.
Tian B, Shin H, Liu S, Fei M, Mu Z, Liu C, Pan Y, Sun Y, Goddard WA III, Ding M. Double-exchange-induced in situ conductivity in nickel-based oxyhydroxides: an effective descriptor for electrocatalytic oxygen evolution. Angew Chem Int Ed. 2021;60(30):16448. https://doi.org/10.1002/anie.202101906.
Li Z, Yang J, Chen Z, Zheng C, Wei LQ, Yang Y, Hu H, Wu M, Hu Z. V “bridged” Co–O to eliminate charge transfer barriers and drive lattice oxygen oxidation during water-splitting. Adv Funct Mater. 2021;31(9):2008822. https://doi.org/10.1002/adfm.202008822.
Ramirez AP. Colossal magnetoresistance. J Phys Condens Matter. 1997;9(39):8171. https://doi.org/10.1088/0953-8984/9/39/005.
Li J, Ma J, Du K, Zhao E, Guo J, Mao J, Ling T. Double exchange interaction promoted high-valence metal sites for neutral oxygen evolution reaction. Chem Commun. 2020;56(95):15004. https://doi.org/10.1039/D0CC06453J.
Li J, Pei Q, Wang R, Zhou Y, Zhang Z, Cao Q, Wang D, Mi W, Du Y. Enhanced photocatalytic performance through magnetic field boosting carrier transport. ACS Nano. 2018;12(4):3351. https://doi.org/10.1021/acsnano.7b08770.
Garcés-Pineda FA, Blasco-Ahicart M, Nieto-Castro D, López N, Galán-Mascarós JR. Direct magnetic enhancement of electrocatalytic water oxidation in alkaline media. Nat Energy. 2019;4:519. https://doi.org/10.1038/s41560-019-0404-4.
Chen G, Wan H, Ma W, Zhang N, Cao Y, Liu X, Wang J, Ma R. Layered metal hydroxides and their derivatives: controllable synthesis, chemical exfoliation, and electrocatalytic applications. Adv Energy Mater. 2020;10(11):1902535. https://doi.org/10.1002/aenm.201902535.
González-López J, Cockcroft JK, Fernández-González A, Jimenez A, Grau-Crespo R. Crystal structure of cobalt hydroxide carbonate Co2CO3(OH)2: density functional theory and X-ray diffraction investigation. Acta Crystallogr B Struct Sci Cryst Eng Mater. 2017;73(5):868. https://doi.org/10.1107/S2052520617007983.
Wang SL, Qian LQ, Xu H, Lü GL, Dong WJ, Tang WH. Synthesis and structural characterization of cobalt hydroxide carbonate nanorods and nanosheets. J Alloys Compd. 2009;476(1–2):739. https://doi.org/10.1016/j.jallcom.2008.09.096.
Xie H, Tang S, Zhu J, Vongehr S, Meng X. A high energy density asymmetric all-solid-state supercapacitor based on cobalt carbonate hydroxide nanowire covered N-doped graphene and porous graphene electrodes. J Mater Chem A. 2015;3(36):18505. https://doi.org/10.1039/C5TA05129K.
Liu H, Zhang F, Wang H, Xue J, Guo Y, Qian Q, Zhang G. Oxygen vacancy engineered unsaturated coordination in cobalt carbonate hydroxide nanowires enables highly selective photocatalytic CO2 reduction. Energy Environ Sci. 2021;14(10):5339. https://doi.org/10.1039/D1EE01397A.
Zhu L, Wen Z, Mei W, Li Y, Ye Z. Porous CoO nanostructure arrays converted from rhombic Co(OH)F and needle-like Co(CO3)0.5(OH)·0.11H2O and their electrochemical properties. J Phys Chem C. 2013;117(40):204. https://doi.org/10.1021/jp406146b.
Wu T, Sun S, Song J, Xi S, Du Y, Chen B, Sasangka WA, Liao H, Gan CL, Scherer GG, Zeng L, Wang H, Li H, Grimaud A, Xu ZJ. Iron-facilitated dynamic active-site generation on spinel CoAl2O4 with self-termination of surface reconstruction for water oxidation. Nat Catal. 2019;2(9):763. https://doi.org/10.1038/s41929-019-0325-4.
Liu MF, Du ZZ, Xie YL, Li X, Yan ZB, Liu JM. Unusual ferromagnetism enhancement in ferromagnetically optimal manganite La0.7-yCa0.3+yMn1-yRuyO3 (0≤y<0.3): the role of Mn–Ru t2g super-exchange. Sci Rep. 2015;5(1):9922. https://doi.org/10.1038/srep09922.
Ge J, Chen RR, Ren X, Liu J, Ong SJH, Xu ZJ. Ferromagnetic–antiferromagnetic coupling core–shell nanoparticles with spin conservation for water oxidation. Adv Mater. 2021;33(42):2101091. https://doi.org/10.1002/adma.202101091.
Zhao S, Wang Z, He Y, Jiang H, Harn YW, Liu X, Su C, Jin H, Li Y, Wang S, Shen Q, Lin Z. A robust route to Co2(OH)2CO3 ultrathin nanosheets with superior lithium storage capability templated by aspartic acid-functionalized graphene oxide. Adv Energy Mater. 2019;9(26):1901093. https://doi.org/10.1002/aenm.201901093.
Yuan L, Liu S, Xu S, Yang X, Bian J, Lv C, Yu Z, He T, Huang Z, Boukhvalov DW, Cheng C, Huang Y, Zhang C. Modulation of Volmer step for efficient alkaline water splitting implemented by titanium oxide promoting surface reconstruction of cobalt carbonate hydroxide. Nano Energy. 2021;82:105732. https://doi.org/10.1016/j.nanoen.2020.105732.
Sun H, Miao Y, Wu T, Wang Q. Exfoliation of bimetallic (Ni, Co) carbonate hydroxide nanowires by Ar plasma for enhanced oxygen evolution. Chem Commun. 2020;56(6):872. https://doi.org/10.1039/C9CC08841E.
Li J, Zhou Q, Shen Z, Li S, Pu J, Zhong C, Cao M, Jin X, Zhang H, Wang Y, Ma H. Synergistic effect of ultrafine nano-Ru decorated cobalt carbonate hydroxides nanowires for accelerated alkaline hydrogen evolution reaction. Electrochim Acta. 2020;331:135367. https://doi.org/10.1016/j.electacta.2019.135367.
Li S, Zhang Y, Liu N, Yu C, Lee SJ, Zhou S, Fu R, Yang J, Guo W, Huang H, Lee JS, Wang C, Kim TR, Nordlund D, Pianetta P, Du X, Zhao J, Liu Y, Qiu J. Operando revealing dynamic reconstruction of NiCo carbonate hydroxide for high-rate energy storage. Joule. 2020;4:1. https://doi.org/10.1016/j.joule.2020.01.018.
Gao P, Zeng Y, Tang P, Wang Z, Yang J, Hu A, Liu J. Understanding the synergistic effects and structural evolution of Co(OH)2 and Co3O4 toward boosting electrochemical charge storage. Adv Funct Mater. 2022;32(4):2108644. https://doi.org/10.1002/adfm.202108644.
Zhang X, Yi H, An Q, Song S. Recent advances in engineering cobalt carbonate hydroxide for enhanced alkaline water splitting. J Alloy Compd. 2021;887:161405. https://doi.org/10.1016/j.jallcom.2021.161405.
Moritomo Y, Asamitsu A, Kuwahara H, Tokura Y. Giant magnetoresistance of manganese oxides with a layered perovskite structure. Nature. 1996;380:141. https://doi.org/10.1038/380141a0.
van der Brink J, Khomskii D. Double exchange via degenerate orbitals. Phys Rev Lett. 1999;82(5):1016. https://doi.org/10.1103/PhysRevLett.82.1016.
Acknowledgements
This study was financially supported by the Program B for Outstanding PhD Candidate of Nanjing University (No. 201801B067).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Li, JM., Hong, H. et al. Unraveling role of double-exchange interaction in electrochemical water oxidation by external magnetic field. Rare Met. 43, 289–297 (2024). https://doi.org/10.1007/s12598-023-02464-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-023-02464-0