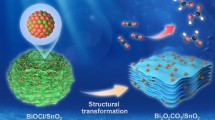
Abstract
Ammonia borane (NH3BH3, AB) has been regarded as a promising chemical hydrogen storage material owing to its high hydrogen density and superior stability. Thus, the development of low-cost and high-efficient heterogeneous catalysts for the dehydrogenation of AB has attracted considerable scholarly attention. In this study, heterostructured Co3O4–SnO2 catalysts containing oxygen vacancy (Vo) with different Co/Sn atomic ratios (designated as Vo–Co–Sn5:x) were synthesized via a simple co-precipitation–calcination method under mild reaction conditions. The catalyst containing an optimized Co/Sn atomic ratio of 5:2 (Vo–Co–Sn5:2) exhibited robust catalytic performance with a turnover frequency value of 17.6 \(\text{mol}_{{\text{H}}_{2}}\)·molmetal−1·min−1. Moreover, 82.6% of the original activity of the catalyst was retained after 14 catalytic cycles, indicating the high stability of the catalyst. Diversified characterization combined with the density functional theory (DFT) calculation confirmed the transfer of electrons from Co3O4 to SnO2 and the distribution of the separated charges on SnO2–Co3O4 interface. The transfer of electrons and the distribution of charges facilitated the adsorption and activation of water on the catalyst, thus accelerating the dissociation of H2O molecule (the rate-determining step of AB hydrolysis). It was found that the Vo adjusted the electron structure of the catalysts rather than acted as active sites. These findings will provide researchers with useful information for designing cheap and highly efficient catalysts for catalytic AB hydrolysis.
Graphical abstract

摘要
氨硼烷(NH3BH3,AB)由于具有较高的氢含量和优越的稳定性,被认为是一种很有前景的化学储氢材料。低成本、高效的多相AB产氢催化剂的开发是实现氨硼烷水解制氢的关键。在本研究中,我们通过简单的共沉淀-煅烧法,合成了不同Co/Sn比例(称为Vo–Co–Sn5:x)的异质结构Co3O4–SnO2催化剂。其中Co/Sn比为5:2(Vo–Co–Sn5:2)的催化剂具有良好的催化性能,TOF为17.6 molH 2•molmetal‒1•min−1。经过14次催化循环后,该催化剂的原有活性保持了82.6%,表明该催化剂的稳定性较高。多种表征结合密度泛函理论计算,证实了电子从Co3O4转移SnO2上,电荷集中分布在SnO2–Co3O4界面上。电子转移和电荷的集中分布促进了H2O在催化剂上的吸附和活化,进而加速了水分子中O-H键的断裂(AB水解的速率决定步骤)。我们发现,Vo调整了催化剂的电子结构,而不是作为活性位点。这些发现将为研究人员设计廉价和高效的催化AB水解催化剂提供有价值的信息。






Similar content being viewed by others
References
Boretti A. Continued fossil fuel emissions and cognition impairment. Int J Global Warm. 2021;24(1):86. https://doi.org/10.1504/IJGW.2021.10037785.
Cui G, Zhang X, Wang H, Li Z, Wang W, Yu Q, Zheng L, Wang Y, Zhu J, Wei M. ZrO2-x modified Cu nanocatalysts with synergistic catalysis towards carbon-oxygen bond hydrogenation. Appl Catal B. 2021;280:119406. https://doi.org/10.1016/j.apcatb.2020.119406.
Longden T, Beck FJ, Jotzo F, Andrews R, Prasad M. “Clean” hydrogen? - Comparing the emissions and costs of fossil fuel versus renewable electricity based hydrogen. Appl Energy. 2022;306:118145. https://doi.org/10.1016/j.apenergy.2021.118145.
Farias CBB, Barreiros RCS, Silva MFD, Casazza AA, Converti A, Sarubbo LA. Use of hydrogen as fuel: a trend of the 21st Century. Energies. 2022;15(1):311. https://doi.org/10.3390/en15010311.
Yang ZX, Li XG, Yao QL, Lu ZH, Zhang N, Xia J, Yang K, Wang YQ, Zhang K, Liu HZ, Zhang LT, Lin HJ, Zhou QJ, Wang F, Yu ZM, Ma JM. 2022 roadmap on hydrogen energy from production to utilizations. Rare Met. 2022;41(10):3251. https://doi.org/10.1007/s12598-022-02029-7.
Olajide SK, Ademola SA, Oladapo O, Cyril OA, James AK. Hydrogen storage materials: a review. Int J Sci Eng Res. 2020;11(9):601. https://doi.org/10.13140/RG.2.2.17270.22087.
Guan S, An L, Chen Y, Liu X, Shi J, Sun Y, Fan Y, Liu B. Enhancing effect of Fe2+ doping of Ni/NiO nanocomposite films on catalytic hydrogen generation. ACS Appl Mater Interfaces. 2021;13:42909. https://doi.org/10.1021/acsami.1c12192.
Feng YF, Zhang XF, Shao YX, Chen XD, Wang HZ, Li JH, Wu M, Dong HF, Liu QB, Li H. Modulating the acidic properties of mesoporous Mox-Ni0.8Cu0.2O nanowires for enhanced catalytic performance toward the methanolysis of ammonia borane for hydrogen production. ACS Appl Mater Interfaces. 2022;14:27979. https://doi.org/10.1021/acsami.2c06234.
Yao QL, Du HX, Lu ZH. Catalytic hydrolysis of ammonia borane for hydrogen production. Prog Chem. 2020;32:1930. https://doi.org/10.7536/PC200323.
Liao JY, Wu Y, Shao YX, Feng YF, Zhang XF, Zhang W, Li H. Ammonia borane methanolysis for hydrogen evolution on Cu3Mo2O9/NiMoO4 hollow microspheres. Chem Eng J. 2022;449:137755. https://doi.org/10.1016/j.cej.2022.137755.
Liu P, Gu X, Kang K, Zhang H, Cheng J, Su H. Highly efficient catalytic hydrogen evolution from ammonia borane using the synergistic effect of crystallinity and size of noble-metal-free nanoparticles supported by porous metal-organic frameworks. ACS Appl Mater Interfaces. 2017;9:10759. https://doi.org/10.1021/acsami.7b01161.
Li X, Zhang C, Luo M, Yao Q, Lu ZH. Ultrafine Rh nanoparticles confined by nitrogen-rich covalent organic frameworks for methanolysis of ammonia borane. Inorg Chem Front. 2020;7:1298. https://doi.org/10.1039/d0qi00073f.
Liu M, Zhou L, Luo X, Wan C, Xu L. Recent advances in noble metal catalysts for hydrogen production from ammonia borane. Catalysts. 2020;10(7):788. https://doi.org/10.3390/catal10070788.
Tunca N, Rakap M. Preparation and characterization of Ni-M (M: Ru, Rh, Pd) nanoclusters as efficient catalysts for hydrogen evolution from ammonia borane methanolysis. Renew Energ. 2020;155:1222. https://doi.org/10.1016/j.renene.2020.04.079.
Chandra M, Xu Q. A high-performance hydrogen generation system: transition metal-catalyzed dissociation and hydrolysis of ammonia-borane. J Power Sources. 2006;156(2):190. https://doi.org/10.1016/j.jpowsour.2005.05.043.
Li M, Zhang S, Zhao J, Wang H. Maximizing metal support interactions in Pt/Co3O4 nanocages to simultaneously boost hydrogen production activity and durability. ACS Appl Mater Interfaces. 2021;13:57362. https://doi.org/10.1021/acsami.1c18403.
Wang C, Zhao J, Du X, Sun S, Yang X. Hydrogen production from ammonia borane hydrolysis catalyzed by non-noble metal-based materials: a review. J Mater Sci. 2021;56(4):1. https://doi.org/10.1007/s10853-020-05493-7.
Yao QL, Ding Y, Lu ZH. Noble-metal-free nanocatalysts for hydrogen generation from boron-and nitrogen-based hydrides. Inorg Chem Front. 2020;7(20):3837. https://doi.org/10.1039/D0QI00766H.
Ye C, Li LL, Shu Y, Li QR, Xia J, Hou ZP, Zhou Y, Liu XX, Yang YY, Zhao GP. Generation and manipulation of skyrmions and other topological spin structures with rare metals. Rare Met. 2022;41(7):2200. https://doi.org/10.1007/s12598-021-01908-9.
Feng Y, Wang H, Chen X, Lv F, Li Y, Zhu Y, Xu C, Zhang X, Liu HR, Li H. Simple synthesis of Cu2O-CoO nanoplates with enhanced catalytic activity for hydrogen production from ammonia borane hydrolysis. Int J Hydrog Energy. 2020;45:17164. https://doi.org/10.1016/j.ijhydene.2020.04.257.
Feng YF, Chen XD, Wang HZ, Li X, Huang H, Liu Y, Li H. Durable and high performing Ti supported Ni0.4Cu0.6Co2O4 nanoleaflike array catalysts for hydrogen production. Renew Energy. 2021;169:660. https://doi.org/10.1016/j.renene.2021.01.048.
Lu D, Feng Y, Ding Z, Liao J, Zhang X, Liu H, Li H. MoO3-doped MnCo2O4 microspheres consisting of nanosheets: an inexpensive nanostructured catalyst to hydrolyze ammonia borane for hydrogen generation. Nanomaterials. 2019;9:21. https://doi.org/10.3390/nano9010021.
Song J, Gu X, Cao Y, Zhang H. Porous oxygen vacancy-rich V2O5 nanosheets as superior semiconducting supports of nonprecious metal nanoparticles for efficient on-demand H2 evolution from ammonia borane under visible light irradiation. J Mater Chem A. 2019;7:10543. https://doi.org/10.1039/C9TA01674K.
Feng Y, Li Y, Liao Q, Zhang W, Huang Z, Chen X, Li H. Modulation the electronic structure of hollow structured CuO-NiCo2O4 nanosphere for enhanced catalytic activity towards methanolysis of ammonia borane. Fuel. 2023;332:126045. https://doi.org/10.1016/j.fuel.2022.126045.
Guan S, An L, Ashraf S, Zhang L, Li B. Oxygen vacancy excites Co3O4 nanocrystals embedded into carbon nitride for accelerated hydrogen generation. Appl Catal B Environ. 2020;269:118775. https://doi.org/10.1016/j.apcatb.2020.118775.
Li J, Sun W, Gao P, An J, Sun W. Coffee ground derived biochar embedded Ov-NiCoO2 nanoparticles for efficiently catalyzing a boron-hydrogen bond break. Sci Total Environ. 2020;761:144192. https://doi.org/10.1016/j.scitotenv.2020.144192.
Martinovic FL, Deorsola FA, Armandi M, Boelli B, Pirone R. Composite Cu-SSZ-13 and CeO2-SnO2 for enhanced NH3-SCR resistance towards hydrocarbon deactivation. Appl Catal B Environ. 2021;282:119536. https://doi.org/10.1016/j.apcatb.2020.119536.
Guo Y, Zeng L, Xu X, Liu Y, Wang X. Regulating SnO2 surface by metal oxides possessing redox or acidic properties: the importance of active O2-/O22- and acid sites for toluene deep oxidation. Appl Catal A Gen. 2020;605:117755. https://doi.org/10.1016/j.apcata.2020.117755.
Li H, Zhu D, Yang Z, Lu W, Pu Y. The ethanol-sensitive property of hierarchical MoO3-mixed SnO2 aerogels via facile ambient pressure drying. Appl Surf Sci. 2019;489:384. https://doi.org/10.1016/j.apsusc.2019.05.368.
Huang R, Huang S, Chen D, Zhang Q, Le TT, Wang Q, Hu Z, Chen Z. Environmentally benign synthesis of Co3O4-SnO2 heteronanorods with efficient photocatalytic performance activated by visible light. J Colloid Interface Sci. 2019;542:460. https://doi.org/10.1016/j.jcis.2019.01.089.
Pang Y, Zhou J, Yang X, Lan Y, Chen C. Rationally designed Co3O4-SnO2 activated peroxymonosulfate for the elimination of chloramphenicol. Chem Eng J. 2021;20:129401. https://doi.org/10.1016/j.cej.2021.129401.
Wang X, Zhao L, Li X, Mu J, Liu S. Unveiling the promotion effects of CoO on low-temperature NO reduction with CO over an in-situ -established Co3O4-CoO heterostructure. ACS Sustain Chem Eng. 2021;9:6107. https://doi.org/10.1021/acssuschemeng.1c01593.
Xing Y. Synthesis and electrochemical characterization of uniformly-dispersed high loading Pt nanoparticles on sonochemically-treated carbon nanotubes. J Phys Chem B. 2004;108:19255. https://doi.org/10.1021/jp046697i.
Li Y, Wang S, Wu J, Ma J, Cui L, Lu H, Sheng Z. One-step hydrothermal synthesis of hybrid core-shell Co3O4@SnO2-SnO for supercapacitor electrodes. Ceram Int. 2020;46(10):15793. https://doi.org/10.1016/j.ceramint.2020.03.126.
Zhang H, Liu S. Construction of SnO2/Co3O4 n-p heterojunctions by organometallic chemistry-assisted approach. Mater Lett. 2021;285:129108. https://doi.org/10.1016/j.matlet.2020.129108.
Xing C, Liu Y, Su Y, Chen Y, Wu HS, X, Wang X, Cao H, Li B. Structural evolution of Co-based metal organic frameworks in pyrolysis for synthesis of core-shells on nanosheets: Co@CoOx@carbon-rGO composites for enhanced hydrogen generation activity. ACS Appl Mater Interfaces. 2016;8(24):15430. https://doi.org/10.1021/acsami.6b04058.
Bai S, Liu H, Luo R, Chen A, Li D. SnO2@Co3O4 p-n heterostructures fabricated by electrospinning and mechanism analysis enhanced acetone sensing. RSC Adv. 2014;4:62862. https://doi.org/10.1039/c4ra09766a.
Liu Y, Ma C, Zhang Q, Wang W, Dai Z. 2D electron gas and oxygen vacancy induced high oxygen evolution performances for advanced Co3O4/CeO2 nanohybrids. Adv Mater. 2019;31(21):1900062. https://doi.org/10.1002/adma.201900062.
Yang S, Liu Y, Hao Y, Yang X, Cao B. Oxygen-vacancy abundant ultrafine Co3O4/graphene composites for high-rate supercapacitor electrodes. Adv Sci. 2018;5(4):1700659. https://doi.org/10.1002/advs.201700659.
Verma R, Samdarshi S, Bojja S, Paul S, Choudhury B. A novel thermophotocatalyst of mixed-phase cerium oxide (CeO2/Ce2O3) homocomposite nanostructure: role of interface and oxygen vacancies. Sol Energy Mater Sol Cells. 2015;141:414. https://doi.org/10.1016/j.solmat.2015.06.027.
Ruiz Puigdollers A, Schlexer P, Tosoni S, Pacchioni G. Increasing oxide reducibility: the role of metal/oxide interfaces in the formation of oxygen vacancies. Acs Catal. 2017;7(10):6493. https://doi.org/10.1021/acscatal.7b01913.
Aidhy D, Liu B, Zhang Y, Weber W. Strain-induced phase and oxygen-vacancy stability in ionic interfaces from first-principles calculations. J Phys Chem C. 2014;118(51):30139. https://doi.org/10.1021/jp507876m.
Tang X, Hao J, Li J. Complete oxidation of methane on Co3O4-SnO2 catalysts. Front Environ Sci Engin China. 2009;3(3):265. https://doi.org/10.1007/s11783-009-0019-2.
Liao JY, Feng YF, Lin WM, Su XL, Ji S, Li LL, Zhang WL, Pollet BG, Li H. CuO-NiO/Co3O4 hybrid nanoplates as highly active catalyst for ammonia borane hydrolysis. Int J Hydrog Energy. 2020;45(15):8168. https://doi.org/10.1016/j.ijhydene.2020.01.155.
Zhou S, Yang Y, Yin P, Ren Z, Wang L, Wei M. Metal-support synergistic catalysis in Pt/MoO3-x nanorods toward ammonia borane hydrolysis with efficient hydrogen generation. ACS Appl Mater Interfaces. 2022;14(4):5275. https://doi.org/10.1021/acsami.1c20736.
Wang X, Liao J, Li H, Wang H, Wang R, Pollet BG, Ji S. Highly active porous Co-B nanoalloy synthesized on liquid-gas interface for hydrolysis of sodium borohydride. Int J Hydrog Energy. 2018;43(37):17543. https://doi.org/10.1016/j.ijhydene.2018.07.147.
Lu T, Li T, Shi D, Sun J, Pang H, Xu L, Yang J, Tang Y. In situ establishment of Co/MoS2 heterostructures onto inverse opal-structured N, S-doped carbon hollow nanospheres: interfacial and architectural dual engineering for efficient hydrogen evolution reaction. SmartMat. 2021;002(004):591. https://doi.org/10.1002/smm2.1063.
Qin Y, Zhang W, Wang F, Li J, Ye J, Sheng X, Li C, Liang X, Liu P, Wang X, Zheng X, Ren Y, Xu C, Zhang Z. Extraordinary p–d hybridization interaction in heterostructural Pd-PdSe nanosheets boosts C−C bond cleavage of ethylene glycol electrooxidation. Angew Chem Int Ed. 2022;61(16):e202200899. https://doi.org/10.1002/anie.202200899.
Wang F, Zhang W, Wan H, Li C, An W, Sheng X, Liang X, Wang X, Ren Y, Zheng X, Lv D, Qin Y. Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction. Chinese Chem Lett. 2022;33(5):2259. https://doi.org/10.1016/j.cclet.2021.08.074.
Li Z, He T, Liu L, Chen W, Zhang M, Wu G, Chen P. Covalent triazine framework supported non-noble metal nanoparticles with superior activity for catalytic hydrolysis of ammonia borane: from mechanistic study to catalyst design. Chem Sci. 2017;8(1):781. https://doi.org/10.1039/C6SC02456D.
Wang C, Li L, Yu X, Lu Z, Zhang X, Wang X, Yang X, Zhao J. Regulation of d band electrons to enhance the activity of Co-based non-noble bimetal catalysts for hydrolysis of ammonia borane. ACS Sustain Chem Eng. 2020;8(22):8256. https://doi.org/10.1021/acssuschemeng.0c01475.
Liao J, Shao Y, Feng Y, Zhang J, Song C, Zeng W, Li H. Interfacial charge transfer induced dual-active-sites of heterostructured Cu0.8Ni0.2WO4 nanoparticles in ammonia borane methanolysis for fast hydrogen production. Appl Catal B Environ. 2023;320:121973. https://doi.org/10.1016/j.apcatb.2022.121973.
Acknowledgements
This work was financially supported by the Professorial and Doctoral Scientific Research Foundation of Huizhou University (Nos. 2018JB036, 2020JB046 and 2022JB009); the Major and Special Project in the Field of Intelligent Manufacturing of the Universities in Guangdong Province (No. 2020ZDZX2067); the Natural Science Foundation of Huizhou University (No. HZU202004); Open Project Program of Guangdong Provincial Key Laboratory of Electronic Functional Materials and Devices, Huizhou University (Nos. EFMDN2021001Z and EFMDN2021004M); Youth Innovative Talents Project in Colleges and Universities in Guangdong Province (No. 2019KQNCX151). Basic and Applied Basic Research Fund of Guangdong Province (No. 2020A1515110038).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, HZ., Shao, YX., Feng, YF. et al. Heterostructured Co3O4–SnO2 composites containing oxygen vacancy with high activity and recyclability toward NH3BH3 dehydrogenation. Rare Met. 42, 3013–3023 (2023). https://doi.org/10.1007/s12598-023-02305-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-023-02305-0