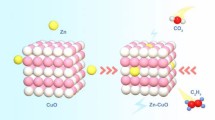
Electrocatalytic reduction of carbon dioxide (CO2RR) into high value-added chemicals and fuels has been regarded as a promising approach to achieve carbon neutrality. Though nickel-nitrogen-carbon (Ni-N-C) electrocatalysts have shown superior CO2RR performance, the synthesis of highly effective Ni-N-C catalyst is still challenging. Herein, a three-dimensional (3D) ordered porous nitrogen-doped carbon-supported Ni-Nx catalyst has been synthesized by direct pyrolysis of a mixture of SiO2, polyvinyl pyrrolidone, nickel-phenanthroline complex, followed by the removal of the SiO2 templates. Benefiting from the porous structure and accessible active sites, the optimized catalyst exhibits a high CO Faradaic efficiency above 85% between –0.6 and –0.9 V versus reversible hydrogen electrode (vs. RHE), and a large CO current density (jCO) of –16.2 mA·cm−2 at –0.8 V (vs. RHE). Density functional theory (DFT) calculations demonstrate that the Ni-N-C catalyst with Ni-Nx species can enhance CO2RR reaction dynamic process and suppress hydrogen evolution reaction, thus improving the conversion efficiency toward CO2RR.
Graphical Abstract

摘要
电催化还原二氧化碳(CO2RR)为高附加值的化学品和燃料,是实现碳中和的一个有前景的途径。尽管镍-氮-碳 (Ni-N-C)电催化剂已显示出不错的二氧化碳还原性能,但高效的Ni-N-C 催化剂的合成仍然是一个挑战。在此,通过直接热解二氧化硅、聚乙烯吡咯烷酮、镍-菲罗啉复合物的混合物,然后去除二氧化硅模板,合成了一种Ni-Nx 位点锚定的三维(3D)有序多孔的氮掺杂碳支撑的电催化剂。受益于多孔结构和可利用的活性位点,优化后的 催化剂在‒0.6 至‒0.9 V(vs. RHE)之间表现出85%以上的高CO 法拉达效率,并且在‒0.8V 下, CO 电流密度 (jCO),达到‒16.2 mA·cm-2。密度泛函理论(DFT)计算表明,含有Ni-Nx 位点锚定的Ni-N-C 催化剂可以促进 CO2RR 过程,同时抑制析氢反应,从而提高CO2RR 催化性能。






Similar content being viewed by others
References
Liu HF, Xia J, Zhang N, Cheng H, Bi WT, Zu XL, Chu WS, Wu HA, Wu CZ, Xie Y. Solid-liquid phase transition induced electrocatalytic switching from hydrogen evolution to highly selective CO2 reduction. Nat Catal. 2021;4(4):202. https://doi.org/10.1038/s41929-021-00608-y.
Li JJ, Zhang ZC. K+-enhanced electrocatalytic CO2 reduction to multicarbon products in strong acid. Rare Met. 2022;41(3):723. https://doi.org/10.1007/s12598-021-01862-6.
Pei J, Wang T, Sui R, Zhang X, Zhou D, Qin F, Zhao X, Liu Q, Yan W, Dong J, Zheng L, Li A, Mao J, Zhu W, Chen W, Zhuang Z. N-Bridged Co–N–Ni: new bimetallic sites for promoting electrochemical CO2 reduction. Energy Environ Sci. 2021;14(5):3019. https://doi.org/10.1039/D0EE03947K.
Liu K, Wang J, Shi M, Yan J, Jiang Q. Simultaneous achieving of high faradaic efficiency and CO partial current density for CO2 reduction via robust, noble-metal-free Zn nanosheets with favorable adsorption energy. Adv Energy Mater. 2019;9(21):1900276. https://doi.org/10.1002/aenm.201900276.
Li Q, Wang YC, Zeng J, Zhao X, Chen C, Wu QM, Chen LM, Chen ZY, Lei YP. Bimetallic chalcogenides for electrocatalytic CO2 reduction. Rare Met. 2021;40(12):3442. https://doi.org/10.1007/s12598-021-01772-7
Yang H, Huang Y, Deng J, Wu Y, Han N, Zha C, Li L, Li Y. Selective electrocatalytic CO2 reduction enabled by SnO2 nanoclusters. J Energy Chem. 2019;37:93. https://doi.org/10.1016/j.jechem.2018.12.004.
Liu XX, Chen C, He Q, Kong Q, Blackwood DJ, Li NW, Yu L, Chen JS. Self-supported transition metal-based nanoarrays for efficient energy storage. Chem Rec. 2022. https://doi.org/10.1002/tcr.202100294.
Yu X, Shao M, Yang X, Li C, Li T, Li D, Wang R, Yin L. A high-performance potassium-ion capacitor based on a porous carbon cathode originated from the Aldol reaction product. Chin Chem Lett. 2020;31(9):2215. https://doi.org/10.1016/j.cclet.2019.11.012.
Abbas M, Sial MAZG. New horizon in stabilization of single atoms on metal-oxide supports for CO2 reduction. Nano Mater Sci. 2021;3(4):368. https://doi.org/10.1016/j.nanoms.2021.07.009.
Yang H, Liu Y, Liu X, Wang X, Tian H, Waterhouse GIN, Kruger PE, Telfer SG, Ma S. Large-scale synthesis of N-doped carbon capsules supporting atomically dispersed iron for efficient oxygen reduction reaction electrocatalysis. eScience. 2022;2(2):227. https://doi.org/10.1016/j.esci.2022.02.005.
Zhang Y, Hu T, Ke C, Han F, Xiao W, Yang X. Ru nanoclusters confined on α/β cobalt hydroxide nanosheets as efficient bifunctional oxygen electrocatalysts for Zn-air batteries. Inorg Chem Front. 2022. https://doi.org/10.1039/d2qi01585d.
Shen S, Han C, Wang B, Wang Y. Engineering d-band center of nickel in nickel@nitrogen-doped carbon nanotubes array for electrochemical reduction of CO2 to CO and ZN-CO2 batteries. Chin Chem Lett. 2021;33(8):3721. https://doi.org/10.1016/j.cclet.2021.10.063.
Cui Y, Zhang Y, Cao Z, Gu J, Du Z, Li B, Yang S. A perspective on high-entropy two-dimensional materials. SusMat. 2022;2(1):65. https://doi.org/10.1002/sus2.47.
Zhang S, Gao XT, Hou PF, Zhang TR, Kang P. Nitrogen-doped Zn–Ni oxide for electrochemical reduction of carbon dioxide in sea water. Rare Met. 2021;40(11):3117. https://doi.org/10.1007/s12598-021-01774-5.
Yin C, Li Q, Zheng J, Ni Y, Wu H, Kjøniksen AL, Liu C, Lei Y, Zhang Y. Progress in regulating electronic structure strategies on Cu-based bimetallic catalysts for CO2 reduction reaction. Adv Powder Mater. 2022;1(4):100055. https://doi.org/10.1016/j.apmate.2022.100055.
Du J, Liu L, Yu Y, Zhang Y, Chen A. “Dissolution-reassembly” for N-doped hollow micro/meso-carbon spheres with high supercapacitor performance. Chin Chem Lett. 2019;30(7):1423. https://doi.org/10.1016/j.cclet.2019.03.004.
Zeng J, Bejtka K, Ju W, Castellino M, Chiodoni A, Sacco A, Farkhondehfal MA, Hernández S, Rentsch D, Battaglia C, Pirri CF. Advanced Cu-Sn foam for selectively converting CO2 to CO in aqueous solution. Appl Catal B Environ. 2018;236:475. https://doi.org/10.1016/j.apcatb.2018.05.056.
Chen Y, Chen K, Fu J, Yamaguchi A, Li H, Pan H, Hu J, Miyauchi M, Liu M. Recent advances in the utilization of copper sulfide compounds for electrochemical CO2 reduction. Nano Mater Sci. 2020;2(3):235. https://doi.org/10.1016/j.nanoms.2019.10.006.
Geng Z, Kong X, Chen W, Su H, Liu Y, Cai F, Wang G, Zeng J. Oxygen vacancies in ZnO nanosheets enhance CO2 electrochemical reduction to CO. Angew Chem Int Ed. 2018;57(21):6054. https://doi.org/10.1002/anie.201711255.
Hu C, Bai S, Gao L, Liang S, Yang J, Cheng SD, Mi SB, Qiu J. Porosity-induced high selectivity for CO2 electroreduction to CO on Fe-doped ZIF-derived carbon catalysts. ACS Catal. 2019;9(12):11579. https://doi.org/10.1021/acscatal.9b03175.
Yang CH, Nosheen F, Zhang ZC. Recent progress in structural modulation of metal nanomaterials for electrocatalytic CO2 reduction. Rare Met. 2021;40(6):1412. https://doi.org/10.1007/s12598-020-01600-4.
Yan D, Zhang L, Shen L, Hu R, Xiao W, Yang X. Pd nanoparticles enbedded in N-enriched MOF-derived architectures for efficient oxygen reduction reaction in alkaline media. Green Energy Environ. 2022. https://doi.org/10.1016/j.gee.2022.01.011.
Zhao L, Wu R, Wang J, Li Z, Wei X, Chen JS, Chen Y. Synthesis of noble metal-based intermetallic electrocatalysts by space-confined pyrolysis: recent progress and future perspective. J Energy Chem. 2021;60:61. https://doi.org/10.1016/j.jechem.2020.12.021.
Wang JJ, Li XP, Cui BF, Zhang Z, Hu XF, Ding J, Deng YD, Han XP, Hu WB. A review of non-noble metal-based electrocatalysts for CO2 lectroreduction. Rare Met. 2021;40(11):3019. https://doi.org/10.1007/s12598-021-01736-x.
Jia Y, Li F, Fan K, Sun L. Cu-based bimetallic electrocatalysts for CO2 reduction. Adv Powder Mater. 2021;1(1):100012. https://doi.org/10.1016/j.apmate.2021.10.003.
Wang J, Zhu Z, Wei X, Li Z, Chen JS, Wu R, Wei Z. Hydrogen-mediated synthesis of 3D hierarchical porous zinc catalyst for CO2 electroreduction with high current density. J Phys Chem C. 2021;125(43):23784. https://doi.org/10.1021/acs.jpcc.1c07498.
Li Z, Wu R, Xiao S, Yang Y, Lai L, Chen JS, Chen Y. Axial chlorine coordinated iron-nitrogen-carbon single-atom catalysts for efficient electrochemical CO2 reduction. Chem Eng J. 2022;430:132882. https://doi.org/10.1016/j.cej.2021.132882.
Zhang Y, Qi K, Li J, Karamoko BA, Lajaunie L, Godiard F, Oliviero E, Cui X, Wang Y, Zhang Y, Wu H, Wang W, Voiry D. 26% cm−2 single-pass CO2-to-CO conversion using Ni single atoms supported on ultra-thin carbon nanosheets in a flow electrolyzer. ACS Catal. 2021;11(20):12701. https://doi.org/10.1021/acscatal.1c03231.
Wei X, Xiao S, Wu R, Zhu Z, Zhao L, Li Z, Wang J, Chen JS, Wei Z. Activating COOH* intermediate by Ni/Ni3ZnC0.7 heterostructure in porous N-doped carbon nanofibers for boosting CO2 electroreduction. Appl Catal B: Environ. 2022;302:120861. https://doi.org/10.1016/j.apcatb.2021.120861.
Li Z, Wu R, Zhao L, Li P, Wei X, Wang J, Chen JS, Zhang T. Metal-support interactions in designing noble metal-based catalysts for electrochemical CO2 reduction: recent advances and future perspectives. Nano Res. 2021;14(11):3795. https://doi.org/10.1007/s12274-021-3363-6.
Wang C, Liu Y, Ren H, Guan Q, Chou S, Li W. Diminishing the uncoordinated N species in Co–N-C catalysts toward highly efficient electrochemical CO2 reduction. ACS Catal. 2022;12(4):2513. https://doi.org/10.1021/acscatal.1c05029.
Duarte M, Daems N, Hereijgers J, Arenas-Esteban D, Bals S, Breugelmans T. Enhanced CO electroreduction with metal-nitrogen-doped carbons in a continuous flow reactor. J CO2 Utili. 2021;50:101583. https://doi.org/10.1016/j.jcou.2021.101583.
Zhu Z, Li Z, Wang J, Li R, Chen H, Li Y, Chen JS, Wu R, Wei Z. Improving NiNX and pyridinic N active sites with space-confined pyrolysis for effective CO2 electroreduction. eScience. 2022;2:445. https://doi.org/10.1016/j.esci.2022.05.002.
Möller T, Ju W, Bagger A, Wang X, Luo F, Ngo TT, Varela AS, Rossmeisl J, Strasser P. Efficient CO2 to CO electrolysis on solid Ni-N-C catalysts at industrial current densities. Energy Environ Sci. 2019;12(2):640. https://doi.org/10.1039/c8ee02662a.
Zhang M, Wu TS, Hong S, Fan Q, Soo YL, Masa J, Qiu J, Sun Z. Efficient electrochemical reduction of CO2 by Ni-N-C catalysts with tunable performance. ACS Sustain Chem Eng. 2019;7(17):15030. https://doi.org/10.1021/acssuschemeng.9b03502.
Zhu Z, Li Z, Wei X, Wang J, Xiao S, Li R, Wu R, Chen JS. Achieving efficient electroreduction of CO2 to CO in a wide potential window by encapsulating Ni nanoparticles in N-doped carbon nanotubes. Carbon. 2021;185:9. https://doi.org/10.1016/j.carbon.2021.08.072.
Li C, Ju W, Vijay S, Timoshenko J, Mou K, Cullen DA, Yang J, Wang X, Pachfule P, Bruckner S, Jeon HS, Haase FT, Tsang SC, Rettenmaier C, Chan K, Cuenya BR, Thomas A, Strasser P. Covalent organic framework (COF) derived Ni-N-C catalysts for electrochemical CO2 reduction: unraveling fundamental kinetic and structural parameters of the active sites. Angew Chem Int Ed. 2022;61(15):e202114707. https://doi.org/10.1002/anie.202114707.
Pan F, Deng W, Justiniano C, Li Y. Identification of champion transition metals centers in metal and nitrogen-codoped carbon catalysts for CO2 reduction. Appl Catal B Environ. 2018;226:463. https://doi.org/10.1016/j.apcatb.2018.01.001.
Wang X, Sang X, Dong CL, Yao S, Shuai L, Lu J, Yang B, Li Z, Lei L, Qiu M, Dai L, Hou Y. Proton capture strategy for enhancing electrochemical CO2 reduction on atomically dispersed metal-nitrogen active sites. Angew Chem Int Ed. 2021;60(21):11959. https://doi.org/10.1002/anie.202100011.
Wang C, Hu X, Hu X, Liu X, Guan Q, Hao R, Liu Y, Li W. Typical transition metal single-atom catalysts with a metal-pyridine N structure for efficient CO2 electroreduction. Appl Catal B Environ. 2021;296:120331. https://doi.org/10.1016/j.apcatb.2021.120331.
Guo H, Si DH, Zhu HJ, Li QX, Huang YB, Cao R. Ni single-atom sites supported on carbon aerogel for highly efficient electroreduction of carbon dioxide with industrial current densities. eScience. 2022;2(3):295. https://doi.org/10.1016/j.esci.2022.03.007.
Leverett J, Yuwono JA, Kumar P, Tran-Phu T, Qu J, Cairney J, Wang X, Simonov AN, Hocking RK, Johannessen B, Dai L, Daiyan R, Amal R. Impurity tolerance of unsaturated Ni-N-C active sites for practical electrochemical CO2 reduction. ACS Energy Lett. 2022;7(3):920. https://doi.org/10.1021/acsenergylett.1c02711.
Yang M, Huang M, Li Y, Feng Z, Huang Y, Chen H, Xu Z, Liu H, Wang Y. Printing assembly of flexible devices with oxidation stable MXene for high performance humidity sensing applications. Sens Actuators B Chem. 2022;364:131867. https://doi.org/10.1016/j.snb.2022.131867.
Wang J, Li Z, Zhu Z, Jiang J, Li Y, Chen J, Niu X, Chen JS, Wu R. Tailoring the interactions of heterostructured Ni4N/Ni3ZnC0.7 for efficient CO2 electroreduction. J Energy Chem. 2022;75:1. https://doi.org/10.1016/j.jechem.2022.07.037.
Zheng W, Wang Y, Shuai L, Wang X, He F, Lei C, Li Z, Yang B, Lei L, Yuan C, Qiu M, Hou Y, Feng X. Highly boosted reaction kinetics in carbon dioxide electroreduction by surface-introduced electronegative dopants. Adv Funct Mater. 2021;31(15):2008146. https://doi.org/10.1002/adfm.202008146.
Lu Q, Chen C, Di Q, Liu W, Sun X, Tuo Y, Zhou Y, Pan Y, Feng X, Li L, Chen D, Zhang J. Dual role of pyridinic-N doping in carbon-coated Ni nanoparticles for highly efficient electrochemical CO2 reduction to CO over a wide potential range. ACS Catal. 2022;12(2):1364. https://doi.org/10.1021/acscatal.1c04825.
Li H, Xiao N, Hao M, Song X, Wang Y, Ji Y, Liu C, Li C, Guo Z, Zhang F, Qiu J. Efficient CO2 electroreduction over pyridinic-N active sites highly exposed on wrinkled porous carbon nanosheets. Chem Eng J. 2018;351:613. https://doi.org/10.1016/j.cej.2018.06.077.
Li Q, Zhu W, Fu J, Zhang H, Wu G, Sun S. Controlled assembly of Cu nanoparticles on pyridinic-N rich graphene for electrochemical reduction of CO2 to ethylene. Nano Energy. 2016;24:1. https://doi.org/10.1016/j.nanoen.2016.03.024.
Ning H, Guo D, Wang X, Tan Z, Wang W, Yang Z, Li L, Zhao Q, Hao J, Wu M. Efficient CO2 electroreduction over N-doped hieratically porous carbon derived from petroleum pitch. J Energy Chem. 2021;56:113. https://doi.org/10.1016/j.jechem.2020.07.049.
Acknowledgements
This study was financially supported by the National Key R&D Program of China (No. 2021YFB2401902).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, SJ., Cheng, H., Yu, J. et al. Three-dimensional ordered porous N-doped carbon-supported accessible Ni-Nx active sites for efficient CO2 electroreduction. Rare Met. 42, 1800–1807 (2023). https://doi.org/10.1007/s12598-022-02247-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-022-02247-z