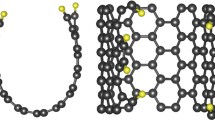
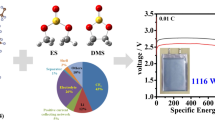
Abstract
Fluorinated graphene has a promising application prospect in lithium primary batteries (LPBs) and sodium primary batteries (SPBs). Herein, five fluorinated graphene materials with different fluorine contents (FG-x) are prepared by a large-scale gas fluorination process. It is found that the structural characteristics of FG-x strongly depend on the fluorination temperature: the fluorine content (i.e., F/C ratio) gradually increases with the fluorination temperature rising, resulting in the enlargement of interlayer spacing and the increase of average bonding strength between C and F. FG-0.75 sample with the intermediate degree of fluorination achieves the maximum energy densities in LPBs (2239.8 Wh·kg−1) and SPBs (1939.2 Wh·kg−1). The interlayer distance is critical to the rate capability of FG-x, and FG-0.95 with the largest lattice spacing exhibits the best rate performance in both Li/CFx and Na/CFx batteries. The electrochemical reaction mechanism and the structural evolution of FG material revealed by ex situ X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD) characterization, and in situ Raman spectra further confirm the effect of interlayer distance.
Graphical abstract

摘要
氟化石墨烯在锂、钠一次电池中具有广阔的应用前景。 本文采用大规模气体氟化法制备了5 种不同氟含量的氟 化石墨烯材料(FG-x)。结果表明,FG-x 的结构特征与氟化温度密切相关:随着氟化温度的升高,氟含量(即F/C 比)逐渐增大,导致了层间距的增大以及C–F 键结合能的增加。中间样品FG-0.75 在LPBs (2239.8 Wh·kg−1)和 SPBs (1939.2 Wh·kg−1)中均表现出最大的能量密度。层间距对FG-x 的倍率性能至关重要,晶格间距最大的FG- 0.95 在Li/CFx 和Na/CFx 电池中均表现出最佳的倍率性能。通过非原位XPS、XRD 和原位拉曼光谱揭示了FG 材 料的电化学反应机理和结构演变,进一步证实了层间距离的影响。






Similar content being viewed by others
References
Jayasinghe R, Thapa AK, Dharmasena RR, Nguyen TQ, Pradhan BK, Paudel HS, Jasinski JB, Sherehiy A, Yoshio M, Sumanasekera GU. Optimization of multi-walled carbon nanotube based CFx electrodes for improved primary and secondary battery performances. J Power Sources. 2014;253:404. https://doi.org/10.1016/j.jpowsour.2013.12.076e.
Huang SZ, Li Y, Feng YY, An HR, Long P, Qin QC, Feng W. Nitrogen and fluorine co-doped graphene as a high-performance anode material for lithium-ion batteries. J Mater Chem A. 2015;3(46):23095. https://doi.org/10.1039/c5ta06012e.
Sideris PJ, Yew R, Nieves I, Chen K, Jain G, Schmidt CL, Greenbaum SG. Charge transfer in Li/CFx–silver vanadium oxide hybrid cathode batteries revealed by solid state 7Li and 19F nuclear magnetic resonance spectroscopy. J Power Sources. 2014;254:293. https://doi.org/10.1016/j.jpowsour.2013.12.108.
Rangasamy E, Li J, Sahu G, Dudney N, Liang CD. Pushing the theoretical limit of Li-CFx batteries: a tale of bifunctional electrolyte. J Am Chem Soc. 2014;136(19):6874. https://doi.org/10.1021/ja5026358.
Wang D, Cai P, Zou GQ, Hou HS, Ji XB, Tian Y, Long Z. Ultra-stable carbon-coated sodium vanadium phosphate as cathode material for sodium-ion battery. Rare Met. 2022;41(1):115. https://doi.org/10.1016/j.jpowsour.2009.02.007.
Li Q, Xue WR, Sun XR, Yu XQ, Li H, Chen LQ. Gaseous electrolyte additive BF3 for high-power Li/CFx primary batteries. Energy Storage Mate. 2021;38:482. https://doi.org/10.1016/j.ensm.2021.03.024.
Huang M, Liu JX, Huang P, Hu H, Lai C. Self-assembly synthesis of SnNb2O6/amino-functionalized graphene nanocomposite as high-rate anode materials for sodium-ion batteries. Chem Soc Rev Rare Metals. 2021;40(2):425. https://doi.org/10.1039/C6CS00776G.
Che HY, Chen SL, Xie YY, Wang H, Amine K, Liao XZ, Ma ZF. Electrolyte design strategies and research progress for room-temperature sodium-ion batteries. Energ Environ Sci. 2017;10(5):1075. https://doi.org/10.1039/C7EE00524E.
Wei CH, Guo S, Wen Ma, Mei SX, Xiang B, Gao B. Recent progress of bismuth-based electrode materials for advanced sodium ion batteries anode. Chin J Rare Met. 2021;45(5):611. https://doi.org/10.1039/C5TA09867J.
Yang TZ, Niu XY, Qian T, Shen XW, Zhou JQ, Xu N, Yan CL. Half and full sodium-ion batteries based on maize with high-loading density and long-cycle life. Nanoscale. 2016;8(34):15497. https://doi.org/10.1039/C6NR04424G.
Li YY, Wu XZ, Liu C, Wang S, Zhou PF, Zhou T, Miao ZC, Xing W, Zhuo SP, Zhou J. Fluorinated multi-walled carbon nanotubes as cathode materials of lithium and sodium primary batteries: effect of graphitization of carbon nanotubes. J Mater Chem A. 2019;7(12):7128. https://doi.org/10.1039/C8TA12074A.
Jiang C, Wang BJ, Wu ZR, Qiu JL, Ding ZP, Zou J, Chen SL, Gao P, Niu XB, Wang LP, Li H. Electrolyte-assisted dissolution-recrystallization mechanism towards high energy density and power density CF cathodes in potassium cell. Nano Energy. 2020;70: 104552. https://doi.org/10.1016/j.nanoen.2020.104552.
Guérin K, Pinheiro J, Dubois M, Fawal Z, Masin F, Yazami R, Hamwi A. Synthesis and characterization of highly fluorinated graphite containing sp2 and sp3 carbon. Chem Mater. 2004;16(9):1786. https://doi.org/10.1021/cm034974c.
Wang L, Li YY, Wang S, Zhou PF, Zhao ZD, Li XW, Zhou J, Zhuo SP. Fluorinated nanographite as a cathode material for lithium primary batteries. ChemElectroChem. 2019;6(8):2201. https://doi.org/10.1002/celc.201900194.
Ahmad Y, Dubois M, Guerin K, Hamwi A, Flahaut E. High energy density of primary lithium batteries working with sub-fluorinated few walled carbon nanotubes cathode. J Alloy Compod. 2017;726:852. https://doi.org/10.1016/j.jallcom.2017.08.001.
Sun CB, Feng YY, Li Y, Qin CQ, Zhang QQ, Feng W. Solvothermally exfoliated fluorographene for high-performance lithium primary batteries. Nanoscale. 2014;6(5):2634. https://doi.org/10.1039/c3nr04609e.
Luo ZY, Wang X, Chen DW, Chang QH, Xie SH, Ma ZS, Lei WX, Pan JA, Pan Y, Huang JY. Ultrafast Li/fluorinated graphene primary batteries with high energy density and power density. ACS Appl Mater Inter. 2021;13(16):18809. https://doi.org/10.1021/acsami.1c02064.
Tang Q, Zhou Z, Chen ZF. Graphene-related nanomaterials: tuning properties by functionalization. Nanoscale. 2013;5(11):4541. https://doi.org/10.1039/C3NR33218G.
Zhang SS, Foster D, Wolfenstine J, Read J. Electrochemical characteristic and discharge mechanism of a primary Li/CFx cell. J Power Sources. 2009;187(1):233. https://doi.org/10.1016/j.jpowsour.2008.10.076.
Peng C, Kong LC, Li Y, Fu HY, Sun LD, Feng YY, Feng W. Fluorinated graphene nanoribbons from unzipped single-walled carbon nanotubes for ultrahigh energy density lithium-fluorinated carbon batteries. Sci China Mater. 2021;64:1367. https://doi.org/10.1007/s40843-020-1551-x.
Liu W, Guo R, Wang Y, Dang GJ, Li Y, Sun YT, Huang P, Pei HJ, Lu JC, Xie JY. A low-overpotential sodium/fluorinated graphene battery based on silver nanoparticles as catalyst. J Colloid Interf Sci. 2020;565:70. https://doi.org/10.1016/j.jcis.2020.01.011.
Chen XY, Fan K, Liu Y, Li Y, Liu XY, Feng W, Wang X. Recent advances in fluorinated graphene from synthesis to applications: critical review on functional chemistry and structure engineering. Adv Mater. 2022;34(1):2101665. https://doi.org/10.1002/adma.202101665.
Disa E, Dubois M, Guérin K, Kharbache H, Masin F, Hamwi A. The effect of nanostructure on the thermal properties of fluorinated carbon nanofibres. Carbon. 2011;49(14):4801. https://doi.org/10.1016/j.carbon.2011.06.092.
Mazanek V, Jankovsky O, Luxa J, Sedmidubsky D, Janousek Z, Sembera F, Mikulics M, Sofer Z. Tuning of fluorine content in graphene: towards large-scale production of stoichiometric fluorographene. Nanoscale. 2015;7(32):13646. https://doi.org/10.1039/C5NR03243A.
Li Y, Feng W. The tunable electrochemical performances of carbon fluorides/manganese dioxide hybrid cathodes by their arrangements. J Power Sources. 2015;274:1292. https://doi.org/10.1016/j.jpowsour.2014.10.150.
Nakajima T, Hagiwara R, Moriya K, Watanabe N. Discharge characteristics of poly (carbon monofluoride) prepared from the residual carbon obtained by thermal decomposition of poly (dicarbon monofluoride) and graphite oxide. J Electrochem Soc. 1986;133(9):1761. https://doi.org/10.1149/1.2109014.
Matsuo Y, Inamoto J, Yamamoto H, Matsumoto K, Hagiwara R. Discharge characteristic of fluorinated graphene-like graphite as a cathode of lithium primary battery. Electrochemistry. 2020;88:437. https://doi.org/10.5796/electrochemistry.20-64068.
Chen T, Wang X, Liu Y, Li B, Cheng Z, Wang ZM, Lai WC, Liu XY. Effects of the oxygenic groups on the mechanism of fluorination of graphene oxide and its structure. Phys Chem Chem Phys. 2017;19(7):5504. https://doi.org/10.1039/c6cp07665c.
Bi X, Li YY, Qiu ZP, Liu C, Zhou T, Zhuo SP, Zhou J. Fluorinated graphene prepared by direct fluorination of N, O-doped graphene aerogel at different temperatures for lithium primary batteries. Materials. 2018;11(7):1072. https://doi.org/10.3390/ma11071072.
Delabarre C, Dubois M, Giraudet J, Guérin K, Hamwi A. Electrochemical performance of low temperature fluorinated graphites used as cathode in primary lithium batteries. Carbon. 2006;44(12):2543. https://doi.org/10.1016/j.carbon.2006.05.013.
Guérin K, Yazami R, Hamwi A. Hybrid-type graphite fluoride as cathode material in primary lithium batteries. Electrochem Solid St. 2004;7(6):A159. https://doi.org/10.1149/1.1718244.
Chen J, Yao BW, Li C, Shi GQ. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon. 2013;64:225. https://doi.org/10.1016/j.carbon.2013.07.055.
Yoo MJ, Park HB. Effect of hydrogen peroxide on properties of graphene oxide in Hummers method. Carbon. 2019;141:515. https://doi.org/10.1016/j.carbon.2018.10.009.
Atef O, Al-Othman JAS. Phosphate rock treatment with citric acid for the rapid potentiometric determination of fluoride with ion-selective electrode. Talanta. 2000;51:993. https://doi.org/10.1016/s0039-9140(99)00361-6.
Wilfred WS. The determination of fluorine. Ind Eng Chem. 1924;16(7):703. https://doi.org/10.1039/C5NR03243A.
Yoshida K, Sugawara Y, Saitoh M, Matsumoto K, Hagiwara R, Matsuo Y, Kuwabara A, Ukyo Y, Ikuhara Y. Microscopic characterization of the C–F bonds in fluorine–graphite intercalation compounds. J Power Sources. 2020;445:227320. https://doi.org/10.1016/j.jpowsour.2019.227320.
Yue HJ, Zhang W, Liu HD, Liu ZG, Zhong GM, Yang Y. Synthesis and characterization of fluorinated carbon nanotubes for lithium primary batteries with high power density. Nanotechnology. 2013;24(42):424003. https://doi.org/10.1088/0957-4484/24/42/424003.
Chamssedine F, Dubois M, Guérin K, Giraudet J, Masin F, Ivanov DA, Vidal L, Yazami R, Hamwi A. Reactivity of carbon nanofibers with fluorine gas. Chem Mater. 2007;19(2):161. https://doi.org/10.1021/cm061731m.
Yang L, Zhang YC, Cheng Z, Huang BY, Liu XY. Aligned fluorinated single-walled carbon nanotube as transmission channel towards attenuation of broadband electromagnetic wave. J Mater Chem C. 2018;6(35):9399. https://doi.org/10.1039/c8tc02522c.
Luo ZY, Chen DW, Wang X, Huang JY, Pan Y, Lei WX, Pan JA. Accordion-like fluorinated graphite nanosheets with high power and energy densities for wide-temperature, long shelf-life sodium/potassium primary batteries. Small. 2021;17(20):2008163. https://doi.org/10.1002/smll.202008163.
Sonibare OO, Haeger T, Foley SF. Structural characterization of Nigerian coals by X-ray diffraction, Raman and FTIR spectroscopy. Energy. 2010;35(12):5347. https://doi.org/10.1016/j.energy.2010.07.025.
Nakajima T, Koh M, Gupta V, Žemva B, Lutar K. Electrochemical behavior of graphite highly fluorinated by high oxidation state complex fluorides and elemental fluorine. Electrochim Acta. 2000;45(10):1655. https://doi.org/10.1016/S0013-4686(99)00389-8.
Cheng L, Jandhyala S, Mordi G, Lucero AT, Huang J, Azcatl A, Addou R, Wallace RM, Colombo L, Kim J. Partially fluorinated graphene: structural and electrical characterization. ACS Appl Mater Inter. 2016;8(7):5002. https://doi.org/10.1021/acsami.5b11701.
Inagaki M, Kang F. Graphene derivatives: graphane, fluorographene, graphene oxide, graphyne and graphdiyne. J Mater Chem A. 2014;2(33):13193. https://doi.org/10.1039/C4TA01183J.
Fan K, Fu JM, Liu XK, Liu Y, Lai WC, Liu XY, Wang X. Dependence of the fluorination intercalation of graphene toward high-quality fluorinated graphene formation. Chem Sci. 2019;10(21):5546. https://doi.org/10.1039/C9SC00975B.
Acik M, Yagneswaran S, Peng W, Lee G, Lund BR, Smith DW, Chabal YJ. Spectroscopic evaluation of out-of-plane surface vibration bands from surface functionalization of graphite oxide by fluorination. Carbon. 2014;77:577. https://doi.org/10.1016/j.carbon.2014.05.062.
Li YR, Bruck AM, Brady AB, Bock D, Takeuchi KJ, Takeuchi ES, Marschilok AC. Hybrid Ag2VO2PO4/CFx as a high capacity and energy cathode for primary batteries. J Electrochem Soc. 2017;164(12):A2457. https://doi.org/10.1149/2.0991712jes.
Wang X, Dai YY, Gao J, Huang JY, Li BY, Fan C, Yang J, Liu XY. High-yield production of highly fluorinated graphene by direct heating fluorination of graphene-oxide. ACS Appl Mater Inter. 2013;5(17):8294. https://doi.org/10.1021/am402958p.
Meduri P, Chen H, Chen X, Xiao J, Gross ME, Carlson TJ, Zhang JG, Deng ZD. Hybrid CFx–Ag2V4O11 as a high-energy, power density cathode for application in an underwater acoustic microtransmitter. Electrochem Commun. 2011;13:1344. https://doi.org/10.1016/j.elecom.2011.08.006.
Lai WC, Xu DZ, Wang X, Wang ZM, Liu Y, Zhang XJ, Liu XY. Characterization of the thermal/thermal oxidative stability of fluorinated graphene with various structures. Phys Chem Chem Phys. 2017;19(29):19442. https://doi.org/10.1039/C7CP03684A.
Sato Y, Itoh K, Hagiwara R, Fukunaga T, Ito Y. On the so-called “semi-ionic” C–F bond character in fluorine–GIC. Carbon. 2004;42(15):3243. https://doi.org/10.1016/j.carbon.2004.08.012.
An HR, Li Y, Long P, Gao Y, Qin CQ, Cao C, Feng YY, Feng W. Hydrothermal preparation of fluorinated graphene hydrogel for high-performance supercapacitors. J Power Sources. 2016;312:146. https://doi.org/10.1016/j.jpowsour.2016.02.057.
Watanabe N, Hagiwara R, Nakajima T. On the relation between the overpotentials and structures of graphite fluoride electrode in nonaqueous lithium cell. J Electrochem Soc. 1984;131(9):1980. https://doi.org/10.1149/1.2116004.
Meduri P, Chen H, Xiao J, Martinez JJ, Carlson T, Zhang JG, Deng ZD. Tunable electrochemical properties of fluorinated graphene. J Mater Chem A. 2013;1(27):7866. https://doi.org/10.1039/c3ta11710c.
Li N, He YS, Wang X, Zhang W, Ma ZF, Zhang D. Incorporation of rubidium cations into Li1.2Mn0.54Co0.13Ni0.13O2 layered oxide cathodes for improved cycling stability. Electrochim Acta. 2017;231:363. https://doi.org/10.1016/j.electacta.2017.01.137.
Wang JL, Sun MH, Liu Y, Lin JF, Wang LF, Xu Z, Wang WL, Yuan ZZ, Liu JC, Bai XD. Unraveling nanoscale electrochemical dynamics of graphite fluoride by in situ electron microscopy: key difference between lithiation and sodiation. J Mater Chem A. 2020;8(12):6105. https://doi.org/10.1039/C6TA07919A.
Zhu YL, Zhang LJ, Zhao HH, Fu Y. Significantly improved electrochemical performance of CFx promoted by SiO2 modification for primary lithium batteries. J Mater Chem A. 2017;5(2):796. https://doi.org/10.1039/c6ta07919a.
Liu W, Zhan BX, Shi B, Li Y, Guo R, Pei HJ, Xie JY. Sandwich–like structure of carbon fluoride/graphene oxide/polyacrylonitrile cathode for lithium and sodium batteries. ChemElectroChem. 2017;4(2):436. https://doi.org/10.1016/j.carbon.2018.09.037.
Mar M, Dubois M, Guérin K, Batisse N, Simon B, Bernard P. Tuning fluorine and oxygen distribution in graphite oxifluorides for enhanced performances in primary lithium battery. Carbon. 2019;141:6. https://doi.org/10.1016/j.jfluchem.2019.109369.
Mar M, Dubois M, Guérin K, Batisse N, Simon B, Bernard P. High energy primary lithium battery using oxidized sub-fluorinated graphite fluorides. J Fluorine Chem. 2019;227:109369. https://doi.org/10.1039/c6nr04084e.
Sayahpour B, Hirsh H, Bai S, Schorr NB, Lambert TN, Mayer M, Bao W, Cheng D, Zhang M, Leung K, Harrison KL, Li W, Meng YS. Revisiting discharge mechanism of CFx as a high energy density cathode material for lithium primary battery. Adv Energy Mater. 2021. https://doi.org/10.1038/nmat1846.
Share K, Cohn AP, Cartera RE, Pint CL. Mechanism of potassium ion intercalation staging in few layered graphene from in situ Raman spectroscopy. Nanoscale. 2016;8:16435. https://doi.org/10.1039/c6nr04084e.
Pisana S, Lazzeri M, Casiraghi C, Novoselov KS, Geim AK, Ferrari AC, Mauri F. Breakdown of the adiabatic Born-Oppenheimer approximation in graphene. Nat Mater. 2007;6(3):198. https://doi.org/10.1002/aenm.201900579.
Baddour-Hadjean R, Pereira-Ramos JP. Raman microspectrometry applied to the study of electrode materials for lithium batteries. Chem Rev. 2008;110:1278. https://doi.org/10.1021/acs.chemmater.0c04676.
Liu JL, Yin TT, Tian BB, Zhang BW, Qian C, Wang ZQ, Zhang LL, Liang P, Chen Z, Yan JX, Fan XF, Lin JY, Chen XH, Huang YZ, Loh KP, Shen ZX. Unraveling the potassium storage mechanism in graphite foam. Adv Energy Mater. 2019;9(22):1900579. https://doi.org/10.1002/aenm.202103196.
Leung K, Schorr NB, Mayer M, Lambert TN, Meng YS, Harrison KL. Edge-propagation discharge mechanism in CFx batteries—a first-principles and experimental study. Chem Mater. 2021;33(5):1760. https://doi.org/10.1039/D0TA00093K.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 22078179) and Taishan Scholar Foundation (No. tsqn201812063). We are particularly grateful to Shandong Zhongshan Photoelectric Materials Co., Ltd. for the help on the fluorination process.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, YY., Liu, C., Chen, L. et al. Multi-layered fluorinated graphene cathode materials for lithium and sodium primary batteries. Rare Met. 42, 940–953 (2023). https://doi.org/10.1007/s12598-022-02155-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-022-02155-2