Abstract
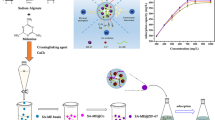
Covalent organic frameworks (COFs) have broad application prospects in adsorption and separation. Yet, as COFs are generally in powder form, their superior performance at the laboratory scale is difficult to transfer into pilot- or industrial-scale use. Thus, there is a strong and urgent need to structure COFs into monolithic materials. Herein, a facile strategy was developed to prepare COFs@alginate composite beads. Three composite beads comprising different COFs including TpPa-1 (2,4,6-triformylphloroglucinol (Tp) and p-phenylenediamine (Pa-1) as monomers), TpDb (Tp and 2,5-diaminobenzonitrile (Db) as monomers) and TpTt (Tp and 1,3,5-triazine-2,4,6-triamine (Tt) as monomers) with controlled COF loading and product size have been facilely achieved via this strategy, validating the applicability of this method. Furthermore, the representative TpDb@alginate composite beads showed good adsorption performance of uranium(VI) in aqueous solution with high adsorption capacity (635 mg·g−1), good interference immunity and recyclability. This work offers a practical approach for incorporation of COFs into polymer matrix, which can serve as potential adsorbents for radioactive wastewater treatment.
Graphical abstract

摘要
共价有机骨架(COFs)在吸附分离方面具有广阔的应用前景。然而, 常规合成的COFs通常是粉末状的, 它们在实验室规模上的优异性能很难转化为工业规模的使用。因此, 急需将COFs制备成整体材料。本文开发了一种简便的COFs@海藻酸复合微珠的制备方法。 通过这种策略, 可以容易地获得三种不同COFs的复合微珠, 包括TpPa-1(2,4,6-三甲酰基间苯三酚(Tp)和对苯二胺(Pa-1)作为单体), TpDb(Tp和2,5-二氨基苯腈(Db)作为单体)和TpTt(Tp和1,3,5-三嗪-2,4,6-三胺(Tt)作为单体), 而且微珠的尺寸和COFs的含量是可以调控的。此外, TpDb@海藻酸盐复合微珠对水溶液中的铀表现出良好的吸附性能, 吸附容量高达635 mg·g-1, 且具有很强的抗干扰能力以及可回收利用性。本工作为制备COFs与聚合物复合吸附剂材料提供了一条切实可行的途径。






Similar content being viewed by others
References
Shan Z, Wu X, Xu B, Hong YL, Wu M, Wang Y, Nishiyama Y, Zhu J, Horike S, Kitagawa S, Zhang G. Dynamic transformation between covalent organic frameworks and discrete organic cages. J Am Chem Soc. 2020;142(51):21279.
Ding SY, Wang W. Covalent organic frameworks (COFs): from design to applications. Chem Soc Rev. 2013;42(2):548.
She P, Qin Y, Wang X, Zhang Q. Recent progress in external-stimulus-responsive 2D covalent organic frameworks. Adv Mater. 2021. https://doi.org/10.1002/adma.202101175.
Wang J, Zhuang S. Covalent organic frameworks (COFs) for environmental applications. Coord Chem Rev. 2019;400:213046.
He L, Chen L, Dong X, Zhang S, Zhang M, Dai X, Liu X, Lin P, Li K, Chen C, Pan T, Ma F, Chen J, Yuan M, Zhang Y, Chen L, Zhou R, Han Y, Chai Z, Wang S. A nitrogen-rich covalent organic framework for simultaneous dynamic capture of iodine and methyl iodide. Chem. 2021;7(3):699.
Du Y, Yang H, Whiteley JM, Wan S, Jin Y, Lee SH, Zhang W. Ionic covalent organic frameworks with spiroborate linkage. Angew Chem Int Ed. 2016;55(5):1737.
Yang Y, Faheem M, Wang L, Meng Q, Sha H, Yang N, Yuan Y, Zhu G. Surface pore engineering of covalent organic frameworks for ammonia capture through synergistic multivariate and open metal site approaches. ACS Cent Sci. 2018;4(6):748.
Pramudya Y, Mendoza-Cortes JL. Design principles for high H2 storage using chelation of abundant transition metals in covalent organic frameworks for 0–700 bar at 298 K. J Am Chem Soc. 2016;138(46):15204.
Zeng Y, Zou R, Zhao Y. Covalent organic frameworks for CO2 capture. Adv Mater. 2016;28(15):2855.
Zhi Y, Wang Z, Zhang HL, Zhang Q. Recent progress in metal-free covalent organic frameworks as heterogeneous catalysts. Small. 2020;16(24):2001070.
Sun Q, Aguila B, Perman J, Nguyen N, Ma S. Flexibility matters: cooperative active sites in covalent organic framework and threaded ionic polymer. J Am Chem Soc. 2016;138(48):15790.
Lin DY, Duan P, Yang WT, Huang XJ, Zhao YJ, Wang CT, Pan QH. Facile fabrication of melamine sponge@covalent organic framework composite for enhanced degradation of tetracycline under visible light. Chem Eng J. 2022;430:132817.
Yu F, Liu W, Li B, Tian D, Zuo JL, Zhang Q. Photostimulus-responsive large-area two-dimensional covalent organic framework films. Angew Chem Int Ed. 2019;58(45):16101.
Yao CJ, Wu Z, Xie J, Yu F, Guo W, Xu ZJ, Li DS, Zhang S, Zhang Q. Two-dimensional (2D) covalent organic framework as efficient cathode for binder-free lithium-ion battery. Chemsuschem. 2020;13(9):2457.
Zhang H, Gu C, Yao MS, Kitagawa S. Hybridization of emerging crystalline porous materials: synthesis dimensionality and electrochemical energy storage application. Adv Energy Mater. 2021. https://doi.org/10.1002/aenm.202100321.
Wu C, Wang X, Zhu T, Li P, Xia S. Covalent organic frameworks embedded membrane via acetic-acid-catalyzed interfacial polymerization for dyes separation: enhanced permeability and selectivity. Chemosphere. 2020;261:127580.
Zhong X, Liang W, Lu Z, Qiu M, Hu B. Ultra-high capacity of graphene oxide conjugated covalent organic framework nanohybrid for U(VI) and Eu(III) adsorption removal. J Mol Liq. 2021;323:114603.
Xu S, Li Z, Zhang L, Zhang W, Li D. In situ growth of COF-rLZU1 on the surface of silica sphere as stationary phase for high performance liquid chromatography. Talanta. 2021;221:121612.
Chen Y, Yang D, Yoon YJ, Pang X, Wang Z, Jung J, He Y, Harn YW, He M, Zhang S, Zhang G, Lin Z. Hairy uniform permanently ligated hollow nanoparticles with precise dimension control and tunable optical properties. J Am Chem Soc. 2017;139(37):12956.
Wang CH, Hu LM, Wang ZF, Zhang M. Electrospun and in situ self-polymerization of polyacrylonitrile containing gadolinium nanofibers for thermal neutron protection. Rare Met. 2018;38(3):252.
Cui BC, Li J, Lin YH, Shen Y, Li M, Deng XL, Nan CW. Polymer-infiltrated layered silicates for dental restorative materials. Rare Met. 2019;38(11):1003.
Chen Y, Wang Z, Harn YW, Pan S, Li Z, Lin S, Peng J, Zhang G, Lin Z. Resolving optical and catalytic activities in thermoresponsive nanoparticles by permanent ligation with temperature-sensitive polymers. Angew Chem Int Ed. 2019;58(34):11910.
Zhang X, Pan S, Song H, Guo W, Zhao S, Chen G, Zhang Q, Jin H, Zhang L, Chen Y, Wang S. Polymer-inorganic thermoelectric nanomaterials: electrical properties, interfacial chemistry engineering, and devices. Front Chem. 2021;9:677821.
Yang S, Peng L, Syzgantseva OA, Trukhina O, Kochetygov I, Justin A, Sun DT, Abedini H, Syzgantseva MA, Oveisi E, Lu G, Queen WL. Preparation of highly porous metal-organic framework beads for metal extraction from liquid streams. J Am Chem Soc. 2020;142(31):13415.
Uyen NTT, Hamid ZAA, Tram NXT, Ahmad N. Fabrication of alginate microspheres for drug delivery: a review. Int J Biol Macromol. 2020;153:1035.
Bian C, Cheng Y, Zhu W, Tong R, Hu M, Gang T. A novel optical fiber mach–zehnder interferometer based on the calcium alginate hydrogel film for humidity sensing. IEEE Sens J. 2020;20(11):5759.
Zhao X, Wang X, Lou T. Preparation of fibrous chitosan/sodium alginate composite foams for the adsorption of cationic and anionic dyes. J Hazard Mater. 2021;403:124054.
Wu G, Jin K, Liu L, Zhang H. A rapid self-healing hydrogel based on PVA and sodium alginate with conductive and cold-resistant properties. Soft Matter. 2020;16(13):3319.
Gong J, He C, Zhang J, Wang L. GO-P25@SA gel beads with excellent separation performance for photocatalytic degradation of rhodamine B. Res Chem Intermed. 2021;47(6):2331.
Nithya Priya V, Rajkumar M, Mobika J, Linto Sibi SP. Alginate coated layered double hydroxide/reduced graphene oxide nanocomposites for removal of toxic As(V) from wastewater. Physica E. 2021;127:114527.
Fu J, Zhu Y, Cheng F, Zhang S, Xiu T, Hu Y, Yang S. A composite chitosan derivative nanoparticle to stabilize a W1/O/W2 emulsion: preparation and characterization. Carbohyd Polym. 2021;256:117533.
Wang D, Li Z, Zhang Q, Liu J, Yang Y, Han J, Wang L. Small things make a big difference: the small-molecule cross-linker of robust water-soluble network binders for stable Si anodes. Chem Res Chin U. 2021;37(2):304.
Song Y, Wang N, Yang LY, Wang YG, Yu D, Ouyang XK. Facile fabrication of ZIF-8/calcium alginate microparticles for highly efficient adsorption of Pb(II) from aqueous solutions. Ind Eng Chem Res. 2019;58(16):6394.
Wang N, Zhang G, Wang L, Li J, An Q, Ji S. Pervaporation dehydration of acetic acid using NH2-UiO-66/PEI mixed matrix membranes. Sep Purif Technol. 2017;186:20.
Yang H, Wu H, Pan F, Li Z, Ding H, Liu G, Jiang Z, Zhang P, Cao X, Wang B. Highly water-permeable and stable hybrid membrane with asymmetric covalent organic framework distribution. J Membrane Sci. 2016;520:583.
Yang H, Cheng X, Cheng X, Pan F, Wu H, Liu G, Song Y, Cao X, Jiang Z. Highly water-selective membranes based on hollow covalent organic frameworks with fast transport pathways. J Membrane Sci. 2018;565:331.
Yang H, Wu H, Pan F, Wang M, Jiang Z, Cheng Q, Huang C. Water-selective hybrid membranes with improved interfacial compatibility from mussel-inspired dopamine-modified alginate and covalent organic frameworks. Chin J Chem Eng. 2020;28(1):90.
Li ZD, Zhang HQ, Xiong XH, Luo F. U(VI) adsorption onto covalent organic frameworks-TpPa-1. J Solid State Chem. 2019;277:484.
Yuan M, Wang X, Chen L, Zhang M, He L, Ma F, Liu W, Wang S. Tailoring pore structure and morphologies in covalent organic frameworks for Xe/Kr capture and separation. Chem Res Chin U. 2021;37(3):679.
Sun Q, Aguila B, Earl LD, Abney CW, Wojtas L, Thallapally PK, Ma S. Covalent organic frameworks as a decorating platform for utilization and affinity enhancement of chelating sites for radionuclide sequestration. Adv Mater. 2018;30(20):1705479.
Bhadra M, Kandambeth S, Sahoo MK, Addicoat M, Balaraman E, Banerjee R. Triazine functionalized porous covalent organic framework for photo-organocatalytic E-Z isomerization of olefins. J Am Chem Soc. 2019;141(15):6152.
Yu Y, He Y, Mu Z, Zhao Y, Kong K, Liu Z, Tang R. Biomimetic mineralized organic–inorganic hybrid macrofiber with spider silk-like supertoughness. Adv Funct Mater. 2019;30(6):1908556.
Cho Y, Kim J, Elabd A, Choi S, Park K, Kwon TW, Lee J, Char K, Coskun A, Choi JW. A pyrene-poly(acrylic acid)-polyrotaxane supramolecular binder network for high-performance silicon negative electrodes. Adv Mater. 2019;31(51):1905048.
Ma K, Li P, Xin JH, Chen Y, Chen Z, Goswami S, Liu X, Kato S, Chen H, Zhang X, Bai J, Wasson MC, Maldonado RR, Snurr RQ, Farha OK. Ultrastable mesoporous hydrogen-bonded organic framework-based fiber composites toward mustard gas detoxification. Cell Rep Phys Sci. 2020;1(2):10024.
Zhong X, Liang W, Lu Z, Hu B. Highly efficient enrichment mechanism of U(VI) and Eu(III) by covalent organic frameworks with intramolecular hydrogen-bonding from solutions. Appl Surf Sci. 2020;504:144403.
Wang F, Chen Z, Yang W, Liu L, Ren G, Liu Y, Pan Q. Preparation and adsorption performance for U(VI) of ZnO@ZIF-8 core@shell microspheres. Chem J Chin Univer. 2019;40(1):24.
Liu L, Yang W, Gu D, Zhao X, Pan Q. In situ preparation of chitosan/ZIF-8 composite beads for highly efficient removal of U(VI). Front Chem. 2019;7:607.
Qin X, Yang W, Yang Y, Gu D, Guo D, Pan Q. A zinc metal-organic framework for concurrent adsorption and detection of uranium. Inorg Chem. 2020;59(14):9857.
Zhou L, Liu J, Liu Z. Adsorption of platinum(IV) and palladium(II) from aqueous solution by thiourea-modified chitosan microspheres. J Hazard Mater. 2009;172(1):439.
Zhang YD, Luo XG, Huang ST, Wang J, Zong YL, Zhou J, Ou MH. A sorbent of expanded rice husk powder for removal of uranyl ion from aqueous solution. Rare Met. 2016;35(5):425.
Wang D, Song J, Lin S, Wen J, Ma C, Yuan Y, Lei M, Wang X, Wang N, Wu H. A marine-inspired hybrid sponge for highly efficient uranium extraction from seawater. Adv Funct Mater. 2019;29(32):1901009.
Gok C, Aytas S. Biosorption of uranium(VI) from aqueous solution using calcium alginate beads. J Hazard Mater. 2009;168(1):369.
Yi X, He J, Guo Y, Han Z, Yang M, Jin J, Gu J, Ou M, Xu X. Encapsulating Fe3O4 into calcium alginate coated chitosan hydrochloride hydrogel beads for removal of Cu(II) and U(VI) from aqueous solutions. Ecotox Environ Safe. 2018;147:699.
Yin J, Yang S, He W, Zhao T, Li C, Hua D. Biogene-derived aerogels for simultaneously selective adsorption of uranium(VI) and strontium(II) by co-imprinting method. Sep Purif Technol. 2021;271:118849.
Bai Z, Liu Q, Zhang H, Yu J, Chen R, Liu J, Song D, Li R, Wang J. Anti-biofouling and water-stable balanced charged metal organic framework-based polyelectrolyte hydrogels for extracting uranium from seawater. ACS Appl Mater Inter. 2020;12(15):18012.
He YR, Li XL, Li XL, Tan ZY, Zhang D, Chen HB. Aerogel based on melamine-formaldehyde and alginate: simply removing of uranium from aqueous solutions. J Mol Liq. 2019;289:111154.
Jiang X, Wang H, Wang Q, Hu E, Duan Y. Immobilizing amino-functionalized mesoporous silica into sodium alginate for efficiently removing low concentrations of uranium. J Clean Prod. 2020;247:119162.
Acknowledgements
This study was financially supported by the Natural Science Foundation of Hainan Province (No. 2019RC005), the National Natural Science Foundation of China (Nos. 22061014 and 21761010) and Hainan University Start-Up Fund (No. KYQD(ZR)1806).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Duan, P., Lin, DY., Yang, WT. et al. Facile preparation of covalent organic frameworks@alginate composite beads for enhanced uranium(VI) adsorption. Rare Met. 41, 1323–1331 (2022). https://doi.org/10.1007/s12598-021-01884-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-021-01884-0