Abstract
A double acid corrosion and subsequent hydrothermal treatment were used to fabricate a micro–nano-structured Ti substrates (Ti–M–N). Afterward, the mesoporous polydopamine (MPDA) nanoparticles as photothermal agent were prepared and immobilized on the surface of Ti–M–N samples, in order to obtain Ti–M–N-MPDA sample. Unique micro–nanostructure properties and the photothermal effect of the modified Ti implant caused physical stress on the bacteria and the bacterial membrane damage, and eventually led to bacteria death. More importantly, based on excellent bioactivity and cytocompatibility of mussel-inspired materials, MPDA promoted adhesion, proliferation and osteogenic differentiation of mesenchymal stem cells in vitro. Furthermore, animal experiments in vivo further confirmed that the modified Ti implants could enhance osseointegration.
Graphic abstract

摘要
使用双酸腐蚀和随后的水热处理来制造微纳米结构的 Ti 衬底 (Ti-M-N)。 随后, 制备了作为光热剂的介孔聚多巴胺 (MPDA) 纳米颗粒并将其固定在 Ti-M-N 样品的表面, 以获得 Ti-M-N-MPDA 样品。 独特的微纳米结构特性和改性钛植入物的光热效应对细菌造成物理压力和细菌膜损伤, 最终导致细菌死亡。 更重要的是, 基于贻贝材料优异的生物活性和细胞相容性, MPDA在体外促进间充质干细胞的粘附、增殖和成骨分化。 此外, 体内动物实验进一步证实, 改性钛植入物可以增强骨整合







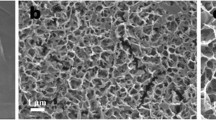
taken from different rats; c antibacterial rate of Ti + NIR and Ti-M-N-MPDA + NIR implants (**p < 0.01)


Similar content being viewed by others
References
Wu XY, Wang T, Zhou M, Huang WJ, Huang WX. Phytic acid/hydroxide hydroxide surface modification on biomineralization properties of 3D printed porous titanium. Chin J Rare Metals. 2020;44(7):680.
Tan L, Li J, Liu XM, Cui ZD, Yang XJ, Zhu SL, Li ZY, Yuan XB, Zheng YF, Yeung KWK, Pan HB, Wang XB, Wu SL. Rapid biofilm eradication on bone implants using red phosphorus and near-infrared light. Adv Mater. 2018;30(31):1801808.
Zhu YP, Li CY, Zhang LY. Corrosion resistance and antibacterial activity of different zones in TA2 weldment by TIG welding. Rare Met. 2020;39(12):1449.
Ding XK, Duan S, Ding XJ, Liu RH, Xu FJ. Versatile antibacterial materials: an emerging arsenal for combatting bacterial pathogens. Adv Funct Mater. 2018;28(40):1802140.
Zhang JM, Sun YH, Zhao Y, Liu YL, Yao XH, Tang B, Hang RQ. Antibacterial ability and cytocompatibility of Cu-incorporated Ni–Ti–O nanopores on NiTi alloy. Rare Met. 2019;38(6):552.
Natalio F, Andre R, Hartog AF, Stoll B, Jochum KP, Wever R, Tremel W. Vanadium pentoxide nanoparticles mimic vanadium haloperoxidases and thwart biofilm formation. Nat Nanotechnol. 2012;7(8):530.
Han J, Yang Y, Lu JR, Wang CZ, Xie YT, Zheng XB, Yao ZJ, Zhang C. Sustained release vancomycin-coated titanium alloy using a novel electrostatic dry powder coating technique may be a potential strategy to reduce implant-related infection. Biosci Trends. 2017;11(3):346.
He Y, Zhang YY, Shen XK, Tao BL, Liu J, Yuan Z, Cai KY. The fabrication and in vitro properties of antibacterial polydopamine-LL-37-POPC coatings on micro-arc oxidized titanium. Colloids Surf B. 2018;170(1):54.
Liu Y, Busscher HJ, Zhao BR, Li YF, Zhang ZK, Van DMH, Ren YJ, Shi LQ. Surface-adaptive, antimicrobially loaded, micellar nanocarriers with enhanced penetration and killing efficiency in staphylococcal biofilms. ACS Nano. 2016;10(4):4779.
Panacek A, Kvitek L, Smekalova M, Vecerova R, Kolar M, Roderova M, Dycka F, Sebela M, Prucek R, Tomanec O, Zboril R. Bacterial resistance to silver nanoparticles and how to overcome it. Nat Nanotechnol. 2018;13(1):65.
Chen M, Zhang Y, Fu S, Yang L, Wang XY, Wang Q, Qin GW, Chen DF, Zhang EL. Effect of fluorination/oxidation level of nano-structured titanium on the behaviors of bacteria and osteoblasts. Appl Surf Sci. 2020;502(1):144077.
Chen WZ, Shen XK, Hu Y, Xu K, Ran QC, Yu YL, Dai LL, Yuan Z, Huang L, Shen TT, Cai KY. Surface functionalization of titanium implants with chitosan-catechol conjugate for suppression of ROS-induced cells damage and improvement of osteogenesis. Biomaterials. 2017;114:82.
Xie XZ, Mao CY, Liu XM, Zhang YZ, Cui ZD, Yang XJ, Yeung K, Pan HB, Chu PK, Wu SL. Synergistic bacteria killing through photodynamic and physical actions of graphene oxide/Ag/collagen coating. ACS Appl Mater Interfaces. 2017;9(31):26417.
Karaman D, Ercan UK, Bakay E, Topaloğlu N, Rosenholm J. Evolving technologies and strategies for combating antibacterial resistance in the advent of the postantibiotic era. Adv Funct Mater. 2020;30(15):1908783.
Mao CY, Xiang YM, Liu XM, Cui ZD, Yang XJ, Yeung KWK, Pan HB, Wang XB, Chu PK, Wu SL. Photo-inspired antibacterial activity and wound healing acceleration by hydrogel embedded with Ag/Ag@AgCl/ZnO nanostructures. ACS Nano. 2017;11(9):9010.
Jain R, Faith N, Milkowski A, Nelson K, Busche D, Lynn D, Czuprynski C, Abbott N. Using chemoattractants to lure bacteria to contact-killing surfaces. Angew Chem Int Ed. 2016;55(19):5698.
Yin WY, Yu J, Lv FT, Yan L, Zheng LR, Gu ZJ, Zhao YL. Functionalized nano-MoS2 with peroxidase catalytic and near-infrared photothermal activities for safe and synergetic wound antibacterial applications. ACS Nano. 2016;10(12):11000.
Cheng L, Wang C, Feng L, Yang K, Liu Z. Functional nanomaterials for phototherapies of cancer. Chem Rev. 2014;114(21):10869.
Kim SH, Kang EB, Jeong CJ, Sharker SM, In I, Park SY. Light controllable surface coating for effective photothermal killing of bacteria. ACS Appl Mater Interfaces. 2015;7(28):15600.
Li Y, Liu XM, Tan L, Cui ZD, Yang XJ, Zheng YF, Yeung KWK, Chu PK, Wu SL. Rapid sterilization and accelerated wound healing using Zn2+ and graphene oxide modified g-C3N4 under dual light irradiation. Adv Funct Mater. 2018;28(30):1800299.
Wei T, Yu Q, Chen H. Antibacterial coatings: responsive and synergistic antibacterial coatings: fighting against bacteria in a smart and effective way. Adv Healthcare Mater. 2019;3(8):1801381.
Yang YC, He P, Wang YX, Bai HT, Shu X. Xu FJ, Supramolecular radical anions triggered by bacteria in situ for selective photothermal therapy. Angew Chem Int Ed. 2017;56(51):16239.
Fasciani C, Silvero MJ, Anghel MA, Argüello GA, Becerra MC, Scaiano JC. Aspartame-stabilized gold-silver bimetallic biocompatible nanostructures with plasmonic photothermal properties, antibacterial activity, and longterm stability. J Am Chem Soc. 2014;136(50):17394.
Ji MW, Xu M, Zhang W, Yang ZZ, Huang L, Liu JJ, Zhang Y, Gu L, Yu YX, Hao WC, An P, Zheng LR, Zhu HS, Zhang JT. Structurally well-defined Au@Cu2-xS core-shell nanocrystals for improved cancer treatment based on enhanced photothermal efficiency. Adv Mater. 2016;28(16):3094.
Xiao LH, Sun JH, Liu LB, Hu R, Lu H, Cheng CG, Huang Y, Wang S, Geng JX. Enhanced photothermal bactericidal activity of the reduced graphene oxide modified by cationic water-soluble conjugated polymer. ACS Appl Mater Interfaces. 2017;9(6):5382.
Li XN, Robinson SM, Gupta A, Saha K, Jiang ZW, Moyano DF, Sahar A, Riley MA, Rotello VM. Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano. 2014;8(10):10682.
Kennedy J, Blair IS, McDowell DA, Bolton DJ. An investigation of the thermal inactivation of Staphylococcus aureus and the potential for increased thermotolerance as a result of chilled storage. Appl Microbiol. 2010;99(5):1229.
Yang Y, Ma L, Cheng C, Deng YY, Huang JB, Fan X, Nie CX, Zhao WF, Zhao CS. Nonchemotherapic and robust dual responsive nanoagents with on-demand bacterial trapping, ablation, and release for efficient wound disinfection. Adv Funct Mater. 2018;21(28):1705708.
Yang Y, Zhu WJ, Dong ZL, Chao Y, Xu L, Chen MW, Liu Z. 1D coordination polymer nanofibers for low-temperature photothermal therapy. Adv Mater. 2017;29(40):1.
Korupalli C, Huang CC, Lin WC, Pan WY, Lin PY, Wan WL, Li MJ, Chang Y, Sung HW. Acidity-triggered charge-convertible nanoparticles that can cause bacterium-specific aggregation in situ to enhance photothermal ablation of focal infection. Biomaterials. 2017;116:1.
Hsiao CW, Chen HL, Liao ZX, Sureshbabu R, Hsiao HC, Lin SJ, Chang Y, Sung HW. Photothermal agents: effective photothermal killing of pathogenic bacteria by using spatially tunable colloidal gels with nano-localized heating sources. Adv Funct Mater. 2015;25(5):721.
Ivanova EP, Hasan J, Webb HK, Truong VK, Watson GS, Watson JA, Baulin VA, Pogodin S, Wang JY, Tobin MJ. Natural bactericidal surfaces: mechanical rupture of pseudomonas aeruginosa cells by cicada wings. Small. 2012;8(16):2489.
Nowlin K, Boseman A, Covell A, Lajeunesse D. Adhesion-dependent rupturing of saccharomyces cerevisiae on biological antimicrobial nanostructured surfaces. J R Soc Interface. 2014;12(102):20140999.
Kelleher S, Habimana O, Lawler J, O’Reilly B, Daniels S, Casey E, Cowley A. Cicada wing surface topography: an investigation into the bactericidal properties of nanostructural features. ACS Appl Mater Interfaces. 2015;8(24):14966.
Linklater DP, Juodkazis S, Rubanov S, Ivanova EP. Commenton “Bactericidal effects of natural nanotopography of dragonfly wing on Escherichia coli”. ACS Appl Mater Interfaces. 2017;9(35):29387.
Tripathy A, Sen P, Su B, Briscoe WH. Natural and bioinspired nanostructured bactericidal surfaces. Adv Colloid Interface Sci. 2017;248:85.
Perreault F, Faria AF, Nejati S, Elimelech M. Antimicrobial properties of graphene oxide nanosheets: why size matters. ACS Nano. 2015;9(7):7226.
Tu YS, Lv M, Xiu P, Huynh T, Zhang M, Castelli M, Liu ZR, Huang Q, Fan CH, Fang HP, Zhou RH. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat Nanotechnol. 2013;8(12):968.
Yuan Z, Tao BL, He Y, Liu J, Lin CC, Shen XK, Ding Y, Yu YL, Mu CY, Liu P, Cai KY. Biocompatible MoS2/PDA-RGD coating on titanium implant with antibacterial property via intrinsic ROS-independent oxidative stress and NIR irradiation. Biomaterials. 2019;217:119290.
Bandara CD, Singh S, Afara IO, Wolff A, Tesfamichael T, Ostrikov K, Oloyede A. Bactericidal effects of natural nanotopography of dragonfly wing on escherichia coli. ACS Appl Mater Interfaces. 2017;9(8):6746.
Zhu C, Bao NR, Chen S, Zhao JN. Antimicrobial design of titanium surface that kill sessile bacteria but support stem cells adhesion. Appl Surf Sci. 2016;389(15):7.
Gao G, Jiang YW, Jia HR, Wu FG. Near-infrared light controllable on-demand antibiotics release using thermo-sensitive hydrogel-based drug reservoir for combating bacterial infection. Biomaterials. 2019;188:83.
Hong S, Na YS, Choi S, Song IT, Kim WY, Lee H. Non-covalent self-assembly and covalent polymerization co-contribute to polydopamine formation. Adv Funct Mater. 2012;22(22):4711.
Xing YX, Zhang JX, Chen F, Liu JJ, Cai KY. Mesoporous polydopamine nanoparticles with co-delivery function for overcoming multidrug resistance via synergistic chemo-photothermal therapy. Nanoscale. 2017;9(25):8781.
Han L, Zhang YN, Lu X, Wang KF, Wang ZM, Zhang HP. Polydopamine nanoparticles modulating stimuli-responsive PNIPAM hydrogels with cell/tissue adhesiveness. ACS Appl Mater Interfaces. 2016;8(42):29088.
Pacelli S, Paolicelli P, Petralito S, Subham S, Gilmore D, Varani G, Yang G, Lin D, Casadei MA, Paul A. Investigating the role of polydopamine to modulate stem cell adhesion and proliferation on gellan gum-based hydrogels. ACS Appl Bio Mater. 2020;2(3):945.
Wang ZQ, Wang LC, Prabhakar N, Xing YX, Rosenholm JM, Zhang JX, Cai KY. CaP coated mesoporous polydopamine nanoparticles with responsive membrane permeation ability for combined photothermal and siRNA therapy. Acta Biomater. 2019;86(1):416.
Shen XK, Ma PP, Hu Y, Xu GQ, Zhou J, Cai KY. Mesenchymal stem cell growth behavior on micro/nano hierarchical surfaces of titanium substrates. Colloids Surf B. 2015;127(1):221.
Lee H, Rho J, Messersmith PB. Facile conjugation of biomolecules onto surfaces via mussel adhesive protein inspired coatings. Adv Mater. 2009;21(4):431.
Wang T, Wan Y, Liu ZQ. Fabrication of hierarchical micro/nanotopography on bio-titanium alloy surface for cytocompatibility improvement. J Mater Sci. 2016;51(21):9551.
Yuan Z, Tao BL, He Y, Mu CY, Lui GH, Zhang JX, Liao Q, Liu P, Cai KY. Remote eradication of biofilm on titanium implant via near-infrared light triggered photothermal/photodynamic therapy strategy. Biomaterials. 2019;223:119479.
Li J, Tan L, Liu XM, Cui ZD, Yang XJ, Yeung KWK, Paul P, Wu SL. Balancing bacteria-osteoblast competition through selective physical puncture and biofunctionalization of ZnO/polydopamine/Arginine-Glycine-Aspartic AcidCysteine nanorods. ACS Nano. 2017;11(11):11250.
Yu S, Li GW, Liu R, Ma D, Xue W. Dendritic Fe3O4@Poly(dopamine)@PAMAM nanocomposite as controllable NO-releasing material: a synergistic photothermal and NO antibacterial study. Adv Funct Mater. 2018;28(20):1707440.
Liu H, Qu X, Tan HQ, Song JL, Lei M, Kim E, Payne GF, Liu CS. Role of polydopamine’s redox-activity on its pro-oxidant, radical-scavenging, and antimicrobial activities. Acta Biomater. 2019;88(1):181.
Lorenzetti M, Dogsa I, Stosicki T, Stopar D, Kalin M, Kobe S, Novak S. The influence of surface modification on bacterial adhesion to titanium-based substrates. ACS Appl Mater Interfaces. 2015;7(3):1644.
Tripathy A, Sen P, Su B, Briscoe W. Natural and bioinspired nanostructured bactericidal surfaces. Adv Colloid Interface Sci. 2017;248(52):85.
Ivanova EP, Hasan J, Webb HK, Gervinskas G, Juodkazis S, Truong VK, Wu AHF, Lamb RN, Baulin VA, Watson GS, Watson JA, Mainwaring DE, Crawford RJ. Bactericidal activity of black silicon. Nat Commun. 2013;4(1):2838.
Modaresifar K, Azizian S, Ganjian M, FratilaApachitei L, Zadpoor A. Bactericidal effects of nanopatterns: a systematic review. Acta Biomater. 2019;83(1):29.
Acknowledgements
This work was financially supported by the State Key Project of Research and Development (Nos. 2016YFC1100300 and 2017YFB0702603) and the National Natural Science Foundation of China (Nos. 51825302, 21734002 and 51673032). The Analytical and Testing Center of Chongqing University is greatly acknowledged for the help with the characterization of materials.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ding, Y., Yuan, Z., Hu, JW. et al. Surface modification of titanium implants with micro–nano-topography and NIR photothermal property for treating bacterial infection and promoting osseointegration. Rare Met. 41, 673–688 (2022). https://doi.org/10.1007/s12598-021-01830-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-021-01830-0