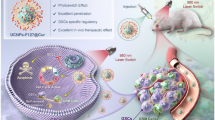
Abstract
To develop smart and efficient near-infrared (NIR) organic dyes for photothermal cancer therapy is of great challenge. Herein, a pH-sensitive NIR dye BTN with donor–acceptor–donor scaffold has been synthesized. Two periphery electron-donating dimethylamine groups in BTN can not only promote the intramolecular photoinduced electron transfer (PET), but also bind protons to transform into electron-deficient ammonium cation for enhanced intramolecular charge transfer (ICT) under acidic circumstance. Through enveloping with amphiphilic polymeric 1,2-distearoyl-ras-glycerol-3-phosphatidyl ethanolamine-N-polyethyleneglycol-2000 (DSPE-mPEG2000), BTN nanoparticles (NPs) were fabricated with robust NIR absorption covering 600–900 nm, excellent photothermal conversion efficiency (43.2%), and good photostability. Additionally, BTN NPs can selectively target lysosomes. Through tailing intravenous injection into tumor-bearing nude mice, BTN NPs demonstrate outstanding photoacoustic/photothermal/fluorescence imaging-guided photothermal therapy for tumor ablation.
Graphic abstract

摘要
开发用于癌症光热治疗的智能、高效型近红外 (NIR) 有机染料至关重要。本文合成了具有供体-受体-供体结构的pH敏感型NIR染料BTN, 其中两侧的给电子性二甲胺基团不仅可以促进分子内光致电子转移 (PET), 而且可以结合质子转化为吸电子性铵根阳离子, 在酸性条件下增强分子内电荷转移 (ICT)。通过两亲性聚合物DSPE-mPEG2000包裹, 制备了在600-900 nm范围内吸收能力强的BTN纳米颗粒 (NPs), 其光热转换性能优异 (43.2%)、光稳定性好。通过尾静脉注射到荷瘤裸鼠体内, BTN NPs表现出优异的光声/光热/荧光成像引导的光热治疗性能。









Similar content being viewed by others
References
Cheng L, Wang C, Feng L, Yang K, Liu Z. Functional nanomaterials for phototherapies of cancer. Chem Rev. 2014;114(21):10869.
Li J, Pu K. Development of organic semiconducting materials for deep-tissue optical imaging, phototherapy and photoactivation. Chem Soc Rev. 2019;48(1):38.
Feng G, Zhang GQ, Ding D. Design of superior phototheranostic agents guided by Jablonski diagrams. Chem Soc Rev. 2020;49(22):8179.
Chen Y, Li L, Chen W, Chen H, Yin J. Near-infrared small molecular fluorescent dyes for photothermal therapy. Chin Chem Lett. 2019;30(7):1353.
Ji C, Cheng W, Yuan Q, Müllen K, Yin M. From Dyestuff Chemistry to cancer theranostics: the rise of rylenecarboximides. Acc Chem Res. 2019;52(8):2266.
Liu S, Zhou X, Zhang H, Ou H, Lam JWY, Liu Y, Shi L, Ding D, Tang BZ. Molecular motion in aggregates: manipulating TICT for boosting photothermal theranostics. J Am Chem Soc. 2019;141(13):5359.
Qi J, Chen C, Zhang X, Hu X, Ji S, Kwok RTK, Lam JWY, Ding D, Tang BZ. Light-driven transformable optical agent with adaptive functions for boosting cancer surgery outcomes. Nat Commun. 2018;9(1):1848.
Gu X, Zhang X, Ma H, Jia S, Zhang P, Zhao Y, Wang J, Zheng X, Lam JWY, Ding D, Tang BZ. Corannulene-incorporated AIE nanodots with highly suppressed nonradiative decay for boosted cancer phototheranostics in vivo. Adv Mater. 2018;30(26):1801065.
Jung HS, Verwilst P, Sharma A, Shin J, Sessler JL, Kim JS. Organic molecule-based photothermal agents: an expanding photothermal therapy universe. Chem Soc Rev. 2018;47(7):2280.
Liu S, Li Y, Kwok RTK, Lam JWY, Tang BZ. Structural and process controls of AIEgens for NIR-II theranostics. Chem Sci. 2021;12(10):3427.
Cai Y, Si W, Huang W, Chen P, Shao J, Dong X. Organic dye based nanoparticles for cancer phototheranostics. Small. 2018;14(25):1704247.
Chen D, Zhong Z, Ma Q, Shao J, Huang W, Dong X. Aza-BODIPY-based nanomedicines in cancer phototheranostics. ACS Appl Mater Interfaces. 2020;12(24):26914.
Li L, Chen Y, Chen W, Tan Y, Chen H, Yin J. Photodynamic therapy based on organic small molecular fluorescent dyes. Chin Chem Lett. 2019;30(10):1689.
Zhou Z, Song J, Nie L, Chen X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem Soc Rev. 2016;45(23):6597.
Chen M, Zhang X, Liu J, Liu F, Zhang R, Wei P, Feng H, Tu M, Qin A, Lam JWY, Ding D, Tang BZ, Lam JWY, Ding D, Zhong Tang B. Evoking photothermy by capturing intramolecular bond stretching vibration-induced dark-state energy. ACS Nano. 2020;14(4):4265.
Zhang Z, Xu W, Kang M, Wen H, Guo H, Zhang P, Xi L, Li K, Wang L, Wang D, Tang BZ. An all-round athlete on the track of phototheranostics: subtly regulating the balance between radiative and nonradiative decays for multimodal imaging-guided synergistic therapy. Adv Mater. 2020;32(36):2003210.
Liang P, Tang Q, Cai Y, Liu G, Si W, Shao J, Huang W, Zhang Q, Dong X. Self-quenched ferrocenyl diketopyrrolopyrrole organic nanoparticles with amplifying photothermal effect for cancer therapy. Chem Sci. 2017;8(11):7457.
Hu W, Miao X, Tao H, Baev A, Ren C, Fan Q, He T, Huang W, Prasad PN. Manipulating nonradiative decay channel by intermolecular charge transfer for exceptionally improved photothermal conversion. ACS Nano. 2019;13(10):12006.
Chen H, Zhao Y. Applications of light-responsive systems for cancer theranostics. ACS Appl Mater Interfaces. 2018;10(25):21021.
Zou J, Zhu J, Yang Z, Li L, Fan W, He L, Tang W, Deng L, Mu J, Ma Y, Cheng Y, Huang W, Dong X, Chen X. A phototheranostic strategy to continuously deliver singlet oxygen in the dark and hypoxic tumor microenvironment. Angew Chem Int Ed. 2020;59(23):8833.
Zhao Z, Chen C, Wu W, Wang F, Du L, Zhang X, Xiong Y, He X, Cai Y, Kwok RTK, Lam JWY, Gao X, Sun P, Phillips DL, Ding D, Tang BZ. Highly efficient photothermal nanoagent achieved by harvesting energy via excited-state intramolecular motion within nanoparticles. Nat Commun. 2019;10(1):768.
Yang X, Liu G, Shi Y, Huang W, Shao J, Dong X. Nano-black phosphorus for combined cancer phototherapy: recent advances and prospects. Nanotechnology. 2018;29(22):222001.
Cheng L, Wang X, Gong F, Liu T, Liu Z. 2D nanomaterials for cancer theranostic applications. Adv Mater. 2020;32(13):1902333.
Liu G, Zou J, Tang Q, Yang X, Zhang Y, Zhang Q, Huang W, Chen P, Shao J, Dong X. Surface modified Ti3C2 MXene nanosheets for tumor targeting photothermal/photodynamic/chemo synergistic therapy. ACS Appl Mater Interfaces. 2017;9(46):40077.
Yang X, Wang D, Shi Y, Zou J, Zhao Q, Zhang Q, Huang W, Shao J, Xie X, Dong X. Black phosphorus nanosheets immobilizing Ce6 for imaging-guided photothermal/photodynamic cancer therapy. ACS Appl Mater Interfaces. 2018;10(15):12431.
Yang X, Yang M, Pang B, Vara M, Xia Y. Gold nanomaterials at work in biomedicine. Chem Rev. 2015;115(19):10410.
Qi J, Fang Y, Kwok RTK, Zhang X, Hu X, Lam JWY, Ding D, Tang BZ. Highly stable organic small molecular nanoparticles as an advanced and biocompatible phototheranostic agent of tumor in living mice. ACS Nano. 2017;11(7):7177.
Qu H, Cui W, Li J, Shao J, Chi C. 6,13-dibromopentacene [2,3:9,10]-bis(dicarboximide): a versatile building block for stable pentacene derivatives. Org Lett. 2011;13(5):924.
Shao J, Wang G, Wang K, Yang C, Wang M. Direct arylation polycondensation for efficient synthesis of narrow-bandgap alternating D-A copolymers consisting of naphthalene diimide as an acceptor. Polym Chem. 2015;6(38):6836.
Li L, Shao C, Liu T, Chao Z, Chen H, Xiao F, He H, Wei Z, Zhu Y, Wang H, Zhang X, Wen Y, Yang B, He F, Tian L. An NIR-II-emissive photosensitizer for hypoxia-tolerant photodynamic theranostics. Adv Mater. 2020;32(45):2003471.
Cheng Z, Zhang T, Wang W, Shen Q, Hong Y, Shao J, Xie X, Fei Z, Dong X. D-A-D structured selenadiazolesbenzothiadiazole-based near-infrared dye for enhanced photoacoustic imaging and photothermal cancer therapy. Chin Chem Lett. 2021;32(4):1582.
Yin Z, Chen D, Zou J, Shao J, Tang H, Xu H, Si W, Dong X. Tumor microenvironment responsive oxygen-self-generating nanoplatform for dual-imaging guided photodynamic and photothermal therapy. ChemistrySelect. 2018;3:4366.
Wang D, Lee MMS, Xu W, Shan G, Zheng X, Kwok RTK, Lam JWY, Hu X, Tang BZ. Boosting non-radiative decay to do useful work: development of a multi-modality theranostic system from an AIEgen. Angew Chem Int Ed. 2019;58(17):5628.
Tang Q, Xiao W, Huang C, Si W, Shao J, Huang W, Chen P, Zhang Q, Dong X. pH-triggered and enhanced simultaneous photodynamic and photothermal therapy guided by photoacoustic and photothermal imaging. Chem Mater. 2017;29(12):5216.
Feng L, Dong Z, Tao D, Zhang Y, Liu Z. The acidic tumor microenvironment: a target for smart cancer nano-theranostics. Natl Sci Rev. 2018;5(2):269.
Chen H, Zhang W, Zhu G, Xie J, Chen X. Rethinking cancer nanotheranostics. Nat Rev Mater. 2017;2(7):17024.
Chen D, Qu X, Shao J, Wang W, Dong X. Anti-vascular nano agents: a promising approach for cancer treatment. J Mater Chem B. 2020;8(15):2990.
Xu Y, Mo R, Qi C, Ren Z, Jia X, Kan Z, Li C, Wang F. Dual-property blue and red emission carbon dots for Fe(III) ions detection and cellular imaging. Rare Met. 2021;40(7):1957.
Chen D, Tang Y, Zhu J, Zhang J, Song X, Wang W, Shao J, Huang W, Chen P, Dong X. Photothermal-pH-hypoxia responsive multifunctional nanoplatform for cancer photo-chemo therapy with negligible skin phototoxicity. Biomaterials. 2019;221:119422.
Liang P, Huang X, Wang Y, Chen D, Ou C, Zhang Q, Shao J, Huang W, Dong X. Tumor-microenvironment-responsive nanoconjugate for synergistic antivascular activity and phototherapy. ACS Nano. 2018;12(11):11446.
Chen C, Tang Y, Ding D. Intramolecular motion-associated biomaterials for image-guided cancer surgery. Smart Mater Med. 2020;1:24.
Gao S, Tang G, Hua D, Xiong R, Han J, Jiang S, Zhang Q, Huang C. Stimuli-responsive bio-based polymeric systems and their applications. J Mater Chem B. 2019;7(5):709.
Tang Y, Xue L, Yu Q, Chen D, Cheng Z, Wang W, Shao J, Dong X. Smart Aza-BODIPY photosensitizer for tumor microenvironment-enhanced cancer phototherapy. ACS Appl Bio Mater. 2019;2(12):5888.
Wang Y, Zhou K, Huang G, Hensley C, Huang X, Ma X, Zhao T, Sumer BD, DeBerardinis RJ, Gao J. A nanoparticle-based strategy for the imaging of a broad range of tumours by nonlinear amplification of microenvironment signals. Nat Mater. 2014;13(2):204.
Horiuchi H, Kuribara R, Hirabara A, Okutsu T. pH-response optimization of amino-substituted tetraphenylporphyrin derivatives as pH-activatable photosensitizers. J Phys Chem A. 2016;120(28):5554.
Tian J, Ding L, Xu HJ, Shen Z, Ju H, Jia L, Bao L, Yu JS. Cell-specific and pH-activatable rubyrin-loaded nanoparticles for highly selective near-infrared photodynamic therapy against cancer. J Am Chem Soc. 2013;135(50):18850.
Shi N, Shi Y, Shao J, Yang X, Zhang X, Zhang Y, Warsame UF, Shao J, Dong X. Selenadiazolobenzotriazole based near infrared dyes with enhanced intramolecular charge transfer and photothermal effect: synthesis, characterization and photophysical properties. Dyes Pigm. 2019;160:683.
Zhang S, Guo W, Wei J, Li C, Liang XJ, Yin M. Terrylenediimide-based intrinsic theranostic nanomedicines with high photothermal conversion efficiency for photoacoustic imaging-guided cancer therapy. ACS Nano. 2017;11(4):3797.
Liu C, Zhang S, Li J, Wei J, Müllen K, Yin M. A water-soluble, NIR-absorbing quaterrylenediimide chromophore for photoacoustic imaging and efficient photothermal cancer therapy. Angew Chem Int Ed. 2019;58(6):1638.
Dai H, Shen Q, Shao J, Wang W, Gao F, Dong X. Small molecular NIR-II fluorophores for cancer phototheranostics. The Innovation. 2021;2(1):100082.
Shao J, Chang J, Dai G, Chi C. Pyromellitic diimide-based copolymers for ambipolar field-effect transistors: synthesis, characterization, and device applications. J Polym Sci, Part A: Polym Chem. 2014;52(17):2454.
Shao J, Chang J, Chi C. Linear and star-shaped pyrazine-containing acene dicarboximides with high electron-affinity. Org Biomol Chem. 2012;10(35):7045.
Wang Z, Peng Q, Huang X, Ma Q, Shen Q, Shao J. Linear acenaphthene-imide-fused pyrazinacene-quinone: synthesis and photophysical properties. Mater Technol. 2021. https://doi.org/10.1080/10667857.2020.1810925.
Shao J, Guo X, Shi N, Zhang X, Liu S, Lin Z, Zhao B, Chang J, Shao J, Dong X. Acenaphthylene-imide based small molecules/TiO2 bilayer as electron-transporting layer for solution-processing efficient perovskite solar cells. Sci China Mater. 2019;62(4):497.
Wang Z, Peng Q, Huang X, Ma Q, Shao J, Shen Q. Recent progress of acenaphthylene-imide-fused polycyclic aromatic hydrocarbons: Synthesis and application. Dyes Pigm. 2021;185:108877.
Zhu W, Kang M, Wu Q, Zhang Z, Wu Y, Li C, Li K, Wang L, Wang D, Tang BZ. Zwitterionic AIEgens: rational molecular design for NIR-II fluorescence imaging-guided synergistic phototherapy. Adv Func Mater. 2020;2007026:2007026.
Li K, Liu B. Polymer-encapsulated organic nanoparticles for fluorescence and photoacoustic imaging. Chem Soc Rev. 2014;43(18):6570.
Qi J, Duan X, Liu W, Li Y, Cai Y, Lam JWY, Kwok RTK, Ding D, Tang BZ. Dragonfly-shaped near-infrared AIEgen with optimal fluorescence brightness for precise image-guided cancer surgery. Biomaterials. 2020;248:120036.
Golombek SK, May JN, Theek B, Appold L, Drude N, Kiessling F, Lammers T. Tumor targeting via EPR: strategies to enhance patient responses. Adv Drug Deliv Rev. 2018;130:17.
Nishi T, Forgac M. The vacuolar (H+)-ATPases-nature’s most versatile proton pumps. Nat Rev Mol Cell Biol. 2002;3(2):94.
Chen D, Tang Q, Zou J, Yang X, Huang W, Zhang Q, Shao J, Dong X. pH-responsive PEG-doxorubicin-encapsulated Aza-BODIPY nanotheranostic agent for imaging-guided synergistic cancer therapy. Adv Healthcare Mater. 2018;7(7):1701272.
Chen D, Yu Q, Huang X, Dai H, Luo T, Shao J, Chen P, Chen J, Huang W, Dong X. A highly-efficient type I photosensitizer with robust vascular-disruption activity for hypoxic-and-metastatic tumor specific photodynamic therapy. Small. 2020;16(23):2001059.
Smith BR, Gambhir SS. Nanomaterials for in vivo imaging. Chem Rev. 2017;117(3):901.
Xiao Y, An F, Chen J, Yu J, Tao W, Yu Z, Ting R, Lee C, Zhang X. The nanoassembly of an intrinsically cytotoxic near-infrared dye for multifunctionally synergistic theranostics. Small. 2019;15(38):1903121.
Song X, Zhang R, Liang C, Chen Q, Gong H, Liu Z. Nano-assemblies of J-aggregates based on a NIR dye as a multifunctional drug carrier for combination cancer therapy. Biomaterials. 2015;57:84.
Yang X, Yu Q, Yang N, Xue L, Shao J, Li B, Shao J, Dong X. Thieno[3,2-b]thiophene-DPP based near-infrared nanotheranostic agent for dual imaging-guided photothermal/photodynamic synergistic therapy. J Mater Chem B. 2019;7(15):2454.
Acknowledgements
The work was financially supported by the Natural Science Foundation of Jiangsu Province (Nos. BK20200092 and BK20200710).
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qu, XY., Hong, Y., Cai, H. et al. Promoted intramolecular photoinduced-electron transfer for multi-mode imaging-guided cancer photothermal therapy. Rare Met. 41, 56–66 (2022). https://doi.org/10.1007/s12598-021-01795-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-021-01795-0