Abstract
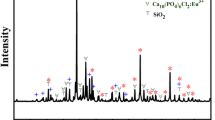
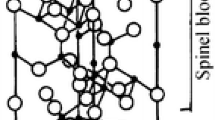
Waste aluminate rare earth phosphor is an important rare earth elements (REEs) secondary resource, which mainly consists of BaMgAl10O17:Eu2+ (BAM) and CeMgAl11O19:Tb3+ (CMAT). Alkaline fusion process is widely used to recycle REEs from aluminate phosphor, but the related theory remains imperfect. In this paper, a series of alkaline fusion experiments of CMAT were performed to describe the phase change law of CMAT reactions. Based on comprehensive analysis, cation–oxoanion synergies theory (COST) was proposed to explain the aluminate phosphor structure damage. On the mirror plane of aluminate phosphor crystal structure, alkali metal cations (Na+, K+) would substitute rare earth ions, while free oxoanion (OH−, CO32−, O22−) can combine with rare earth ions. These two ionic forces ensure that rare earth ions can be substituted by cations. Then, the structure is decomposed. Morphological analysis shows that observable expression of COST can be described by shrinking core model after simplification. Reaction rate constant calculated indicates that the reaction degree is nanometers per second. COST provides a more complete mechanism, and it can help improve rare earth recycling technology furtherly.






Similar content being viewed by others
References
Binnemans K, Jones PT. Rare earths and the balance problem. J Sustain Metall. 2015;1(1):29.
Nakamura E, Sato K. Managing the scarcity of chemical elements. Nat Mater. 2011;10(3):158.
Sun Z, Xiao Y, Agterhuis H, Sietsma J, Yang Y. Recycling of metals from urban mines—a strategic evaluation. J Clean Prod. 2016;12(1):2977.
Mancheri NA. World trade in rare earths, Chinese export restrictions, and implications. Resour Policy. 2015;46(4):262.
Emsbo P, Mclaughlin PI, Breit GN, Bray EAD, Koenig AE. Rare earth elements in sedimentary phosphate deposits: solution to the global REE crisis. Gondwana Res. 2015;27(2):776.
Massari S, Ruberti M. Rare earth elements as critical raw materials: focus on international markets and future strategies. Resour Policy. 2013;38(1):36.
Du X, Graedel TE. Global in-use stocks of the rare earth elements: a first estimate. Environ Sci Technol. 2011;45(9):4096.
Dutta T, Kim KH, Uchimiya M, Kwon EE, Jeon BH, Deep A, Yun ST. Global demand for rare earth resources and strategies for green mining. Environ Res. 2016;150(6):182.
Hatje V, Bruland KW, Flegal AR. Increases in anthropogenic gadolinium anomalies and rare earth element concentrations in San Francisco bay over a 20 year record. Environ Sci Technol. 2016;50(8):4159.
Han W, Li M, Zhang ML, Yan YD. Progress in preparation of rare earth metals and alloys by electrodeposition in molten salts. Rare Met. 2016;35(11):811.
Jüstel T, Nikol H, Ronda C. New developments in the field of luminescent materials for lighting and displays. Angew Chem Int Ed. 1998;37(22):3084.
Rollat A, Guyonnet D, Planchon M, Tuduri J. Prospective analysis of the flows of certain rare earths in Europe at the 2020 horizon. Waste Manag. 2016;49(8):427.
Bandara HMD, Field KD, Emmert MH. Rare earth recovery from end-of-life motors employing green chemistry design principles. Green Chem. 2016;18(3):753.
Shokobayev NM, Bouffier C, Dauletbakov TS. Rare earth metals sorption recovery from uranium in situ leaching process solutions. Rare Met. 2015;34(3):195.
Binnemans K, Jones PT. Perspectives for the recovery of rare earths from end-of-life fluorescent lamps. J Rare Earths. 2014;32(3):195.
Hirajima T, Sasaki K, Bissombolo A, Hirai H, Hamada M, Tsunekawa M. Feasibility of an efficient recovery of rare earth-activated phosphors from waste fluorescent lamps through dense-medium centrifugation. Sep Purif Technol. 2005;44(3):197.
Wu Y, Yin X, Zhang Q, Wang W, Mu X. The recycling of rare earths from waste tricolor phosphors in fluorescent lamps: a review of processes and technologies. Resour Conserv Recycl. 2014;88(2):7927.
Horikawa T, Machida K. Reuse and recycle processing for rare earth phosphors. Mater Integr. 2011;24(5):37.
Innocenzi V, De MI, Ferella F, Veglio F. Recovery of yttrium from cathode ray tubes and lamps’ fluorescent powders: experimental results and economic simulation. Waste Manag. 2013;3(11):2390.
Habib K, Wenzel H. Exploring rare earths supply constraints for the emerging clean energy technologies and the role of recycling. J Clean Prod. 2014;84(1):348.
Zhenyu T. The status quo of rare-earth three primary colors phosphor for lamps. China Light Light. 2012;5(2):006.
Bizarri G, Moine B. On BaMgAl10:Eu2+ phosphor degradation mechanism: thermal treatment effects. J Lumin. 2005;113(3–4):199.
Kim KB, Kim YI, Chun HG, Cho TY, Jung JS, Kang JG. Structural and optical properties of BaMgAl10O17:Eu2+ phosphor. Chem Mater. 2002;14(12):5045.
Wu Z, Cormack A. Defects in BaMgAl10O17:Eu2+ blue phosphor. J Electroceram. 2003;10(3):179.
Zhang J, Zhang Z, Tang Z, Lin Y. Mn2+ luminescence in (Ce, Tb)MgAl11O19 phosphor. Mater Chem Phys. 2001;72(1):81.
Liu H, Zhang SG, Pan DA, Tian JJ, Yang M, Wu ML, Volinsky AA. Rare earth elements recycling from waste phosphor by dual hydrochloric acid dissolution. J Hazard Mater. 2014;272(10):96.
Wu Y, Wang B, Zhang Q, Li R, Yu J. A novel process for high efficiency recovery of rare earth metals from waste phosphors using a sodium peroxide system. RSC Adv. 2014;4(16):7927.
Zhang SG, Liu H, Pan DA, Tian JJ, Liu YF, Volinsky AA. Complete recovery of Eu from BaMgAl10O17:Eu2+ by alkaline fusion and its mechanism. RSC Adv. 2014;5(2):1113.
Liu YF, Zhang SG, Liu H, Pan DA, Liu B, Volinsky AA, Chang C. Free oxoanion theory for BaMgAl10O17:Eu2+ structure decomposition during alkaline fusion process. RSC Adv. 2015;5(62):50105.
Rajak DK, Guria C, Ghosh R, Agarwal S, Pathak AK. Alkali assisted dissolution of fly ash: a shrinking core model under finite solution volume condition. Int J Miner Process. 2016;155(4):106.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Nos. U1360202, 51472030, 51672024 and 515102014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, YF., Zhang, SG., Liu, B. et al. An alkaline fusion mechanism for aluminate rare earth phosphor: cation–oxoanion synergies theory. Rare Met. 38, 299–305 (2019). https://doi.org/10.1007/s12598-017-0898-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-017-0898-5