Abstract
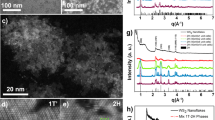
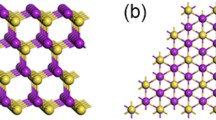
Free-standing two-dimensional (FS-2D) materials of covalent or ionic bonds can be prepared through mechanical exfoliation by virtue of their intrinsic layered crystal structures. Here, following this strategy, it was reported the fabrication of large-area FS-2D Au films with controllable lattice orientations via an electrochemical hydrogen-detaching method. It was further characterized the catalytic properties of FS-2D Au. The (111)-oriented FS-2D Au with 20 nm in thickness shows a catalytic conversion up to 43.8% for the oxidation of cyclohexane as a model reaction system, a result of more than double of generally reported values. The exceptionally high catalytic activity could be attributed to the flexible structure of the FS-2D Au catalyst, which differs from the rigid structure of general catalysts. The results give useful implications for large-scale productions of a variety of FS-2D metals with controllable orientations, by which their applications may not be confined to catalysis chemistry.





Similar content being viewed by others
References
Geim AK. Graphene: status and prospects. Science. 2009;324(5934):1530.
Novoselov KS, Geim AK, Moeozov SV. Two-dimensional gas of massless dirac fermions in grapheme. Nature. 2005;438(7065):197.
Geim AK, Grigorieva IV. Van der waals heterostructures. Nature. 2013;499(7459):419.
Duesberg GS. Heterojunctions in 2D semiconductors: a perfect match. Nat Mater. 2014;13(12):1075.
Koppens FH, Mueller T, Avouris Ph, Ferrari AC, Vitiello MS, Polini M. Photodetectors based on graphene, other two-dimensional materials and hybrid systems. Nat Nanotechnol. 2014;9(10):780.
Yasaei P, Kumar B, Hantehzadeh R, Kayyalha M, Baskin A, Repnin N, Wang CH, Klie RF, Chen YP, Kral P. Chemical sensing with switchable transport channels in graphene grain boundaries. Nat Commun. 2014;5(1):4911.
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA. Electric field effect in atomically thin carbon films. Science. 2004;306(5696):666.
Geim AK, Novoselov KS. The rise of grapheme. Nat Mater. 2007;6(3):183.
Ahmad N, Stokes J, Fox NA. Ultra-thin metal films for enhanced solar absorption. Nano Energy. 2012;1(6):777.
Fekkai Z, Mustapha N, Hennache A. Optical, morphological and electrical properties of silver and aluminium metallization contacts for solar cells. Am J Mod Phys. 2014;3(2):45.
Xu XG, Zhang DL, Wu Y, Zhang X, Li XQ, Yang HL, Jiang Y. Electronic structures of Heusler alloy Co2FeAl1−xSix surface. Rare Met. 2012;31(2):107.
Huang XQ, Tang SH, Mu XL, Dai Y, Chen GX, Zhou ZY, Ruan FX, Yang ZL, Zheng NF. Freestanding palladium nanosheets with plasmonic and catalytic properties. Nat Nanotechnol. 2010;6(1):28.
Iyer GRS, Wang J, Wells G, Guruvenket S, Payne S, Bradley M, Borondics F. Large-area, freestanding, single-layer graphene-gold: a hybrid plasmonic nanostructure. ACS Nano. 2014;8(6):6353.
Hashmi ASK, Hutchings GJ. Gold catalysis. Angew Chem Int Edit. 2006;45(47):7896.
Feng RJ, Li M, Liu JX. Preparation and catalytic activity of CO-resistant catalyst core-shell Au@Pt/C for methanol oxidation. Rare Met. 2012;31(5):451.
Xu ML, Yang XK, Zhang YJ, Xia SB, Dong P, Yang GT. Enhanced methanol oxidation activity of Au@Pd nanoparticles supported on MWCNTs functionalized by MB under ultraviolet irradiation. Rare Met. 2015;34(1):12.
Schuchardt U, Cardoso D, Sercheli R. Cyclohexane oxidation continues to be a challenge. Appl Catal A Gen. 2001;211(1):1.
Sheldon RA, Kochi JK. Metal-Catalyzed Oxidation of Organic Compunds. New York: Academic Press; 1981. 38.
Xu LX, He CH, Zhu MQ. Silica-supported gold catalyst modified by doping with titania for cyclohexane oxidation. Catal Lett. 2007;118(3–4):248.
Lu GM, Zhao R, Qian G, Qi YX, Wang XL, Suo JS. A highly efficient catalyst Au/MCM-41 for selective oxidation cyclohexane using oxygen. Catal Lett. 2004;97(3–4):115.
Süss-Fink G, Gonzalez L, Shul’pin GB. Alkane oxidation with hydrogen peroxide catalyzed homogeneously by vanadium–containing polyphosphomolybdates. Appl Catal A Gen. 2001;217(1–2):111.
Meyer JC, Geim AK, Katsnelson MI. The structure of suspended graphene sheets. Nature. 2007;446(7131):60.
Patil NS, Jha R, Uphade BS. Epoxidation of styrene by anhydrous t-butyl hydroperoxide over gold supported on Al2O3, Ga2O3, In2O3 and Tl2O3. Appl Catal A Gen. 2004;275(1–2):87.
Lee SY, Yamada M, Miyake M. Synthesis of carbon nanotubes over gold nanoparticle supported catalysts. Carbon. 2005;43(13):2654.
Huang XH, Neretina S. Gold nanorods: from synthesis and properties to biological and biomedical applications. Adv Mater. 2009;21(48):4880.
Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004;104(1):293.
Acknowledgements
This study was financially supported by the Fundamental Research Funds for the Central Universities (No. FRF-SD-12-027A), the New Century Excellent Talents in Universities (No. NCET-12-0778), Innovation on Working Methodology of Ministry of Science and Technology of China (No. 2012IM030500), the National Natural Science Foundation of China (No. 51328202) and China Scholarship Council (No. 201308110345).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, SZ., Yang, M., Xiang, QY. et al. Free-standing two-dimensional Au films. Rare Met. 41, 4235–4240 (2022). https://doi.org/10.1007/s12598-016-0827-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-016-0827-z