Abstract
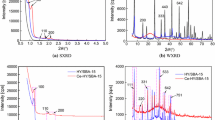
γ-Al2O3-supported CeO2 catalysts were prepared by microemulsion and impregnation methods and characterized by X-ray diffraction (XRD) and scanning electron microscope (SEM) techniques. At the same time, the desulfurization activity of catalysts was investigated. The results show that nanoscale active substances and a high desulfurization effect are achieved by microemulsion, exhibiting a significant dominance compared with traditional impregnation method. The optimal preparation condition is temperature of 30 °C and ratio of [H2O]/[surface active agent] of 7 with slow demulsification. The activated catalysts still keep high and stable desulfurization activity during a wide temperature range of 450–600 °C. Among a series of prepared catalysts, the desulfurization rate of 6CeO2/γ-Al2O3 is the highest, reaching up to 80 % when temperature is higher than 550 °C. The catalytic reduction mechanism of SO2 over nano-CeO2/γ-Al2O3 follows redox mechanism.









Similar content being viewed by others
References
Sudarsanam P, Mallesham B, Reddy PS, Großmann D, Grünert W, Reddy BM. Nano-Au/CeO2 catalysts for CO oxidation: Influence of dopants (Fe, La and Zr) on the physicochemical properties and catalytic activity. Appl Catal B Environ. 2014;144(1):900.
Monjezi BH, Yazdani ME, Mokfi M, Ghiaci M. Liquid phase oxidation of diphenylmethane to benzophenone with molecular oxygen over nano-sized Co-Mn catalyst supported on calcined cow bone. J Mol Catal A Chem. 2014;383–384(3):58.
Wang P, Tang XL, Yi HH, Li K, Wang JG, Xiang Y, Xu XM. Preparation of core-shell structure catalysts and catalytic property for catalytic oxidation of NO at low temperatures. J Cent South Univ T (Nat Sci). 2014;45(1):328.
Liu LC, Zi XH, Dai HX, Zhao Z, Wang XP, He H. Preparation and characterization of Rh-Au/γ-Al2O3 three-way nanocatalysts. Chin J Catal. 2010;31(7):781.
Lee DS, Chen YW. Nano Ag/TiO2 catalyst prepared by chemical deposition and its photocatalytic activity. J Taiwan Inst Chem E. 2014;45(2):705.
Rakmak N, Wiyaratn W, Bunyakan C, Chungsiriporn J. Synthesis of Fe/MgO nano-crystal catalysis by sol-gel method for hydrogen sulfide removal. Chem Eng J. 2010;162(1):84.
Mohamed MS, Mohamed NM. Stack growth of aligned multiwalled carbon nanotubes using floating catalyst chemical vapor deposition technique. Chem Phys Lett. 2015;625(4):53.
Yang GK, Ding KQ. Preparation of Pt-Pd/MWCNTs and Pd/MWCNTs catalysts by hydrothermal synthesis in room temperature ionic liquids. Chem J Chinese U. 2010;31(5):994.
Tavasoli A, Taghavi S. Performance enhancement of bimetallic Co-Ru/CNTs nano catalysts using micro-emulsion technique. J Energy Chem. 2013;22(5):747.
Lu JL, Wang L, Li JD, Wang YY, Li SL, Cui XQ. Pd-Au nano-catalysts synthesized by electrodeposition and its catalytic performance for methanol. Chin J Rare Met. 2015;39(6):493.
Yousefi T, Torab-Mostaedi M, Ghasemi M, Ghadirifar A. Synthesis of Gd2O3 nanoparticles: using bulk Gd2O3 powders as precursor. Rare Met. 2015;34(8):540.
Zolfaghari Z, Tavasoli A, Tabyar S, Pour AN. Enhancement of bimetallic Fe-Mn/CNTs nano catalyst activity and product selectivity using microemulsion technique. J Energy Chem. 2014;23(1):57.
Boutonnet M, Kizling J, Stenius P, Maire G. The preparation of monodisperse colloidal metal particles from microemulsions. Colloids Surf. 1982;5(3):209.
Zhang GJ, Du YN, Zhang YF, Xu Y. Desulfurization reaction model and experimental analysis of high sulfur coal under hydrogen atmosphere. J Ind Eng Chem. 2014;20(2):487.
Han GB, Park NK, Lee TJ. Effect of O2 on SO2 reduction with CO or H2 over SnO2–ZrO2 catalyst. Ind Eng Chem Res. 2009;48(23):10307.
Hu H, Wang SX, Zhang XL, Zhao QZ, Li J. Study on simultaneous catalytic reduction of sulfur dioxide and nitric oxide on rare earth mixed compounds. J Rare Earth. 2006;24(6):695.
Nemade KR, Waghuley SA. Synthesis of CeO2 nanoparticles using flame-assisted spray pyrolysis and solid state diffusion routes. Rare Met. 2015;34(1):6.
Chen J, Meng DQ, Zhang GK, Li SQ, Chen QY. Thickness and optical properties of nanocrystalline CeO2 thin film. Chin J Rare Met. 2014;38(1):28.
Hu H, Wang SX, Zhao QZ, Zhang XL, He ZH, Li J. The function of γ-Al2O3 in catalytic reduction of SO2 to elemental sulfur over CeO2-La2O3 catalysts. Ind Catal. 2006;14(9):59.
Hu H, Li SL, Zhang SX, Li J. Studies on reaction mechanism of SO2 reduction catalyzed by CeO2-La2O3/γ-Al2O3. Chin J Catal. 2004;25(2):115.
Zhou JH, He ZH, Yu FS, Li J, Hu H, Zhao QZ, Wang SX. La2O3–CeO2/γ-Al2O3 catalysts f or CO reduction of SO2 in the presence of oxygen. Environ Pollut Control. 2007;29(2):99.
Zhang JL, Hu H, Wang WC, Wu GM, Wang ZP, Zeng ZW. Catalytic desulfurization and denitration over activated alumina with different sizes supported CeO2–Fe2O3. Environ Eng. 2014;32(10):94.
Sang XL, Li KZ, Wang H, Wei YG. Selective oxidation of methane and carbon deposition over Fe2O3/Ce1−x Zr x O2 oxides. Rare Met. 2014;33(2):230.
Yu CL, Hu JB, Yang K, Liu XY, Fan QZ. Preparation of Ni/M x O y /CeO2–ZrO2 (M = Cu, Ba, Al) composite catalysts and their catalytic performance in methane partial oxidation. Chin J Rare Met. 2014;38(1):60.
Wu X, Tayal J, Basu S, Scott K. Nano-crystalline Ru x Sn1−x O2 powder catalysts for oxygen evolution reaction in proton exchange membrane water electrolysers. Int J Hydrogen Energy. 2011;36(22):14796.
Mosaddegh E. Ultrasonic-assisted preparation of nano eggshell powder: a novel catalyst in green and high efficient synthesis of 2-aminochromenes. Ultrason Sonochem. 2013;20(6):1436.
Machocki A, Denis A, Grzegorczyk W, Gac W. Nano- and micro-powder of zirconia and ceria-supported cobalt catalysts for the steam reforming of bio-ethanol. Appl Surf Sci. 2010;256(7):5551.
Capek I. Preparation of metal nanoparticles in water-in-oil(w/o) microemulsions. Adv Colloid Interface Sci. 2004;110(4):49.
Liu W, Saromfim AF, Stephanopoulos MF. Reduction of sulfur dioxide by carbon monoxide to elemental sulfur over composite oxide catalysts. Appl Catal B. 1994;4(2–3):167.
Lau NT, Fang M, Chan CK. The role of SO2 in the reduction of NO by CO on La2O2S. J Catal. 2007;245(2):301.
Acknowledgments
This study was financially supported by the Natural Science Foundation of Hubei Province, China (No. 2009CDB246) and the Applied Basic Research Project of Wuhan City (No. 2015060101010068).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Q., Hu, H. & Wang, SS. Preparation and desulfurization activity of nano-CeO2/γ-Al2O3 catalysts. Rare Met. 37, 554–560 (2018). https://doi.org/10.1007/s12598-016-0773-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-016-0773-9