Abstract
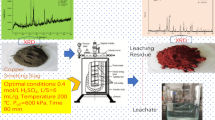
An intensified oxidative acid leaching of copper–cadmium-bearing slag featuring using high-efficient oxygen carrier, such as activated carbon, was investigated to achieve high leaching rate of valuable metals. The effects of leaching variables, including agitation rate, sulfuric acid concentration, temperature, slag particle size, activated carbon and cupric ion concentration, were examined. It is found that leaching rates of cadmium and zinc both exceed 99 % in a very short time, but for copper, leaching rate of 99 % is achieved under the optimized leaching parameters, which are agitation rate of 100 r·min−1, sulfuric acid concentration of 15 wt%, leaching temperature of 80 °C, slag particle size of 48–75 μm, activated carbon concentration of 3 g·L−1, liquid-to-solid ratio of 4:1, oxygen flow rate of 0.16 L·min−1, and leaching time of 60 min. The macro-leaching kinetics of copper metal was analyzed, and it is concluded that the inner diffusion is the controlling step, with apparent activation energy of 18.6 kJ·mol−1. The leaching solution with pH value of 2–4 can be designed to selectively extract valuable metals without neutralization, and the leaching residue can be treated by prevailing Pb smelting process.
Graphical Abstract
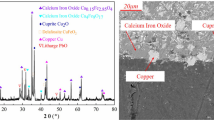
The phase compositions of the leaching residue are PbSO4 and CaSO4. And the chemical composition analysis result shows that about 13 % Pb is contained in the residue, which can be recycled by Pb smelting technology in an Isa furnace. The way is that the Pb-containing concentrate and the PbSO4-containing leaching residue can be mixed and placed in the furnace.

















Similar content being viewed by others
References
Kim T, Senanayake G, Kang J, Sohn J, Rhee K, Lee S, Shin S. Reductive acid leaching of spent zinc–carbon batteries and oxidative precipitation of Mn–Zn ferrite nanoparticles. Hydrometallurgy. 2009;96(1):154.
Babu MN, Sahu K, Pandey B. Zinc recovery from sphalerite concentrate by direct oxidative leaching with ammonium, sodium and potassium persulphates. Hydrometallurgy. 2002;64(2):119.
Li B, Pan DA, Jiang YH, Tian JJ, Zhang SG, Zhang K. Recovery of copper and tin from stripping tin solution by electrodeposition. Rare Met. 2014;33(3):353.
de Souza AD, Pina PS, Leão VA. Bioleaching and chemical leaching as an integrated process in the zinc industry. Miner Eng. 2007;20(6):591.
Wang MY, Wang XW, Jiang CJ, Tao CF. Solvent extraction of molybdenum from acidic leach solution of Ni–Mo ore. Rare Met. 2014;33(1):107.
Ibarra-Galvan V, López-Valdivieso A, Tong X, Cui YQ. Role of oxygen and ammonium ions in silver leaching with thiosulfate–ammonia–cupric ions. Rare Met. 2013;33(2):225.
Shao Q, Du X, Wang L, Lan RZ. Present status of reutilization of copper–cadmium slag. Chin J Hydrometall. 2003;22(2):66.
Ning Q. Study on the recovery of zinc, cadmium, copper from copper–cadmium slag. Chin J Hydrometall. 1998;1:41.
Kasprzyk-Hordern B, Ziółek M, Nawrocki J. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl Catal-B Environ. 2003;46(4):639.
Rivera-Utrilla J, Sánchez-Polo M, Gómez-Serrano V, Álvarez PM, Alvim-Ferraz MCM, Dias JM. Activated carbon modifications to enhance its water treatment applications. An overview. J Hazard Mater. 2011;187(1–3):1.
Stoeckli HF, Rebstein P, Ballerini L. On the assessment of microporosity in active carbons, a comparison of theoretical and experimental data. Carbon. 1990;28(6):907.
Rodríguez-reinoso F. The role of carbon materials in heterogeneous catalysis. Carbon. 1998;36(3):159.
Laine J, Severino F, Labady M. Optimum Ni composition in sulfided Ni–Mo hydrodesulfurization catalysts: effect of the support. J Catal. 1994;147(1):355.
Laine J, Labady M, Severino F, Yunes S. Sink effect in activated carbon-supported hydrodesulfurization catalysts. J Catal. 1997;166(2):384.
Nunez C, Viñals J. Kinetics of leaching of zinc ferrite in aqueous hydrochloric acid solutions. Metall Mater Trans B. 1984;15(2):221.
Elgersma F, Kamst G, Witkamp G, van Rosmalen G. Acidic dissolution of zinc ferrite. Hydrometallurgy. 1992;29(1):173.
Elgersma F, Witkamp GJ, van Rosmalen GM. Kinetics and mechanism of reductive dissolution of zinc ferrite in H2O and D2O. Hydrometallurgy. 1993;33(1–2):165.
Langová Š, Leško J, Matýsek D. Selective leaching of zinc from zinc ferrite with hydrochloric acid. Hydrometallurgy. 2009;95(3):179.
Bobeck GE, Su H. The kinetics of dissolution of sphalerite in ferric chloride solution. Metall Mater Trans B. 1985;16(3):413.
Zhang Y, Li X, Pan L, Liang X, Li X. Studies on the kinetics of zinc and indium extraction from indium-bearing zinc ferrite. Hydrometallurgy. 2010;100(3):172.
Kim J, Kaurich TA, Sylvester P, Gonzalez-Martin A. Enhanced selective leaching of chromium from radioactive sludges. Sep Sci Technol. 2006;41(1):179.
Dutrizac J. The leaching of sulphide minerals in chloride media. Hydrometallurgy. 1992;29(1):1.
Aydogan S, Aras A, Canbazoglu M. Dissolution kinetics of sphalerite in acidic ferric chloride leaching. Chem Eng J. 2005;114(1):67.
Tindall G, Bruckenstein S. Determination of heterogeneous equilibrium constants by chemical stripping at a ring-disk electrode. Evaluation of the equilibrium constant for the reaction copper + copper(II) → 2copper(I) in 0.2 M sulfuric acid. Anal Chem. 1968;40(10):1402.
Mokmeli M, Wassink B, Dreisinger D. Equilibrium cuprous concentrations in copper sulfate–sulfuric acid solutions containing 50–110 g/L Cu2+ and 10–200 g/L H2SO4 at 50–95°C. Hydrometallurgy. 2012;2012(121–124):100.
Ciavatta L, Ferri D, Palombari R. On the equilibrium Cu2+ + Cu(s) ⇄ 2Cu+. J Inorg Nucl Chem. 1980;42(4):593.
Desmarquest JP, Trinh-Dinh C, Bloch O. Determination of normal electrochemical potentials of redox couples reducers Cu2+/Cu+ and Cu+/Cu0 in methanol. J Inorg Nucl Chem. 1970;27(1):101.
Radovic LR. Chemistry and Physics of Carbon. New York: CRC Press; 2000. 121.
Rodrıguez-Reinoso F, Molina-Sabio M. Textural and chemical characterization of microporous carbons. Adv Colloid Interface. 1998;76:271.
Randin J-P, Yeager E. Differential capacitance study on the edge orientation of pyrolytic graphite and glassy carbon electrodes. J Electroanal Chem. 1975;58(2):313.
Derbyshire F, De Beer V, Abotsi G, Scaroni A, Solar J, Skrovanek D. The influence of surface functionality on the activity of carbon-supported catalysts. Appl Catal. 1986;27(1):117.
Boehm H. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon. 1994;32(5):759.
Hossain M, Tryk D, Yeager E. The electrochemistry of graphite and modified graphite surfaces: the reduction of O2. Electrochim Acta. 1989;34(12):1733.
Tatsumi H, Nakase H, Kano K, Ikeda T. Mechanistic study of the autoxidation of reduced flavin and quinone compounds. J Electroanal Chem. 1998;443(2):236.
Ossowski T, Pipka P, Liwo A, Jeziorek D. Electrochemical and UV-spectrophotometric study of oxygen and superoxide anion radical interaction with anthraquinone derivatives and their radical anions. Electrochim Acta. 2000;45(21):3581.
He S, Wang J, Yan J. Pressure leaching of synthetic zinc silicate in sulfuric acid medium. Hydrometallurgy. 2011;108(3):171.
Ryczaj K, Riesenkampf W. Kinetics of the dissolution of zinc-magnesium ferrites in sulphuric acid solutions related to zinc leach processes. Hydrometallurgy. 1983;11(3):363.
Lan ZY, Hu YH, Liu JS, Wang J. Solvent extraction of copper and zinc from bioleaching solutions with LIX984 and D2EHPA. J Cent South Univ Technol. 2005;12(1):45.
Reddy BR, Priya DN. Process development for the separation of copper(II), nickel(II) and zinc(II) from sulphate solutions by solvent extraction using LIX 84 I. Sep Purif Technol. 2005;45(2):163.
Owusu G. Selective extraction of copper from acidic zinc sulfate leach solution using LIX 622. Hydrometallurgy. 1999;51(1):1.
Gupta B, Deep A, Malik P. Extraction and recovery of cadmium using Cyanex 923. Hydrometallurgy. 2001;61(1):65.
Gouvea LR, Morais CA. Recovery of zinc and cadmium from industrial waste by leaching/cementation. Miner Eng. 2007;20(9):956.
Mellah A, Benachour D. The solvent extraction of zinc and cadmium from phosphoric acid solution by di-2-ethylhexyl phosphoric acid in kerosene diluent. Chem Eng Process. 2006;45(8):684.
Pereira DD, Rocha SDF, Mansur MB. Recovery of zinc sulphate from industrial effluents by liquid–liquid extraction using D2EHPA (di-2-ethylhexyl phosphoric acid). Sep Purif Technol. 2007;53(1):89.
He S, Wang J, Yan J. Pressure leaching of high silica Pb–Zn oxide ore in sulfuric acid medium. Hydrometallurgy. 2010;104(2):235.
Acknowledgments
This work was financially supported by the National Science & Technology Pillar Program during the Twelfth Five-Year Plan Period of China (No. 2012BAC12B01) and the Major Scientific and Technological Special Project of Hunan Province, China (No. 2012FJ1010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, M., Zhang, Y., Wang, XH. et al. Extraction of copper, zinc and cadmium from copper–cadmium-bearing slag by oxidative acid leaching process. Rare Met. 40, 1–10 (2021). https://doi.org/10.1007/s12598-016-0759-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-016-0759-7