Abstract
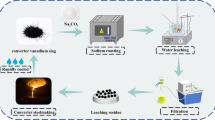
Preparation of V2O5 from converter slag (CS) was investigated through roasting, leaching, extracting, and precipitating processes. The corresponding roles of the parameters during every procedure were analyzed in detail. (NH4)2SO4 and KHSO4 were used as fusing agents to transform compounds containing vanadium into soluble species. Mass ratio of (NH4)2SO4 to CS, mass ratio of KHSO4 to CS, and roasting temperature during the roasting process can significantly influence leaching rate of vanadium (LRV). With H2SO4 as leaching agent, 99.2 % vanadium in CS can be leached out under the optimum leaching conditions, which mainly refer to liquid-to-solid ratio, H2SO4 concentration, leaching temperature, and leaching time. Extracting and back-extracting processes were introduced to purify the vanadium from the H2SO4 lixivium. Extracting rate of vanadium (ERV) greatly depends on iron powder concentration, pH, diisooctyl phosphate (P204) content, volume ratio of extractant to H2SO4 lixivium, and extracting time. By adding ammonium hydroxide, 94.0 % vanadium in back-extracting solution can be separated in the form of precipitates. The product from the roasted precipitate mainly consists of V2O5, the content of which is higher than 90.0 %.







Similar content being viewed by others
References
Moskalyk RR, Alfantazi AM. Processing of vanadium: a review. Miner Eng. 2003;16(9):793.
Liu B, Du H, Wang SN, Zhang Y, Zheng SL, Li LJ, Chen DH. A novel method to extract vanadium and chromium from vanadium slag using molten NaOH–NaNO3 binary system. AIChE J. 2013;59(2):541.
Qiu S, Wei C, Li M, Zhou X, Li C, Deng Z. Dissolution kinetics of vanadium trioxide at high pressure in sodium hydroxide–oxygen systems. Hydrometallurgy. 2011;105(3–4):350.
Lundkvist K, Brämming M, Larsson M, Samuelsson C. System analysis of slag utilisation from vanadium recovery in an integrated steel plant. J Clean Prod. 2013;47:43.
Frank A, Madejb A, Galganc V, Peterssonc LR. Vanadium poisoning of cattle with basic slag. Concentrations in tissues from poisoned animals and from a reference, slaughter-house material. Sci Total Environ. 1996;181(1):73.
Aarabi-Karasgani M, Rashchi F, Mostoufi N, Vahidi E. Leaching of vanadium from LD converter slag using sulfuric acid. Hydrometallurgy. 2010;102(1–4):14.
Xiao Q, Chen Y, Gao Y, Xu H, Zhang Y. Leaching of silica from vanadium-bearing steel slag in sodium hydroxide solution. Hydrometallurgy. 2010;104(2):216.
Li XS, Xie B. Extraction of vanadium from high calcium vanadium slag using direct roasting and soda leaching. Int J Miner Met Mater. 2012;19(7):595.
Liu HB, Du H, Wang DW, Wang SN, Zheng SL, Zhang Y. Kinetics analysis of decomposition of vanadium slag by KOH sub-molten salt method. Trans Nonferrous Met Soc China. 2013;23(5):1489.
Mirazimi SMJ, Rashchi F, Saba M. Vanadium removal from roasted LD converter slag: optimization of parameters by response surface methodology (RSM). Sep Purif Technol. 2013;116:175.
Das B, Prakash S, Reddy PSR, Misra VN. An overview of utilization of slag and sludge from steel industries. Resour Conserv Recyl. 2007;50(1):40.
Voglauer B, Grausam A, Jörgl HP. Reaction-kinetics of the vanadium roast process using steel slag as a secondary raw material. Miner Eng. 2004;17(2):317.
Yu L, Dong YC, Ye GZ, Sichen D. Concentrating of vanadium oxide in vanadium rich phase(s) by addition of SiO2 in converter slag. Ironmak Steelmak. 2007;34(2):131.
Qiu HD, Zhang H, Zhao B, Zhu JF, Liu DR. Dynamics study on vanadium extraction technology from chloride leaching steel slag. Rare Met Mater Eng. 2013;42(4):0696.
Li XS, Xie B, Wang GE, Li XJ. Oxidation process of low-grade vanadium slag in presence of Na2CO3. Trans Nonferrous Met Soc China. 2011;21(8):1860.
Jena BC, Dresler W, Reilly IG. Extraction of titanium, vanadium and iron from titanomagnetite deposits as Pipestone lake, Manitoba, Canada. Miner Eng. 1995;8(1–2):159.
Lozano LJ, Godinez C. Comparative study of solvent extraction of vanadium from sulphate solutions by primene 81R and alamine 336. Miner Eng. 2003;16(3):291.
Bal Y, Bal KE, Cote G. Kinetics of the alkaline stripping of vanadium(V) previously extracted by Aliquat® 336. Miner Eng. 2002;15(5):377.
El-Nadi YA, Awwad NS, Nayl AA. A comparative study of vanadium extraction by Aliquat-336 from acidic and alkaline media with application to spent catalyst. Int J Miner Process. 2009;92(3–4):115.
Lozano LJ, Juan D. Solvent extraction of polyvanadates from sulphate solutions by primene 81R. Its application to the recovery of vanadium from spent sulphuric acid catalysts leaching solutions. Solvent Extr Ion Exch. 2001;19(4):659.
Navarro R, Guzman J, Saucedo I, Revilla J, Guibal E. Vanadium recovery from oil fly ash by leaching, precipitation and solvent extraction processes. Waste Manag. 2007;27(3):425.
Li YG, The Extraction Kinetics of Metal. Beijing: Beijing Atomic Energy Press; 1985. 101.
Zhang Y, Xue XX, Yang H. Synthesis of foliar fertilizer and Ca–S–Si compound fertilizer from titanium-bearing blast furnace slag. J Environ Sci Eng. 2010;4(2):30.
Acknowledgments
This study was financially supported by the Fundamental Research Fund for the Central Universities (No. N130302004), the National Major International Cooperation Program of China (No. 2012DFR60210), the National High-Tech Research and Development Program of China (No. 2012AA062304), and the National Natural Science Foundation of China (Nos. 51090384 and U1360204).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, C., Li, L., Yang, H. et al. Preparation of V2O5 from converter slag containing vanadium. Rare Met. 37, 904–912 (2018). https://doi.org/10.1007/s12598-015-0566-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-015-0566-6