Abstract
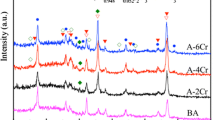
ZrO2 ceramic coatings were directly prepared on the surface of ZrH1.8 in silicate and phosphate electrolytes by micro-arc oxidation (MAO) technique, respectively. The microstructure, chemical composition and phase composition of ZrO2 ceramic coatings were investigated by X-ray diffraction (XRD), energy-dispersive spectrometry (EDS) and scanning electron microscopy (SEM). The anti-permeation effect was measured by means of vacuum dehydrogenation experiment. It is found that the coating fabricated in phosphate electrolyte is more compact than that in silicate electrolyte. The coatings fabricated on the surface of ZrH1.8 are composed of M-ZrO2, T-ZrO2 and C-ZrO2. EDS analysis indicates that the coatings are mainly composed of O and Zr. Vacuum dehydrogenation experiment shows that the permeation reduction factor (PRF) of coating prepared in phosphate electrolyte is superior to that in the silicate electrolyte, and the PRF value reaches up to 11.2, which can enhance the resistance effect of hydrogen significantly.









Similar content being viewed by others
References
Li L, Xu CC, Chen CC, Wang YJ, Jiao LF, Yuan HT. Sodium alanate system for efficient hydrogen storage. Int J Hydrogen Energy. 2013;38(21):8798.
Sakintuna B, Lamari-Darkrim F, Hirscher M. Metal hydride materials for solid hydrogen storage: a review. Int J Hydrogen Energy. 2007;32(9):1121.
Yamanaka S, Yamada K, Kurosaki K, Uno M, Takeda K, Anada H, Matsuda T, Kobayashi S. Analysis of the electronic structure of zirconium hydride. J Alloys Compd. 2002;330–332:313.
Yan GQ, Chen WD, Xue ZQ, Yan SF. Properties of oxide coating on the surface of ZrH1.8 prepared by microarc oxidation with different positive voltages. Rare Met. 2013;32(2):169.
Wongsawaeng D, Jaiyen S. High-temperature absolute hydrogen desorption kinetics of zirconium hydride under clean and oxidized surface conditions. J Nucl Mater. 2010;403(1–3):19.
Szpunar JA, Qin W, Li H, Kumar NAPK. Roles of texture in controlling oxidation, hydrogen ingress and hydride formation in Zr alloys. J Nucl Mater. 2012;427(1–3):343.
Chen WD, Wang LJ, Lu SG. Influence of oxide layer on hydrogen desorption from zirconium hydride. J Alloys Compd. 2009;469(1–2):142.
Chen WD, Yan SF, Zhong XK. Properties of oxide film on the surface of ZrHx (x = 0~2). In: the 11th IUMRS International Conference in Asia (IUMRS-ICA2010), Materials Science Forum. 2011; 686:609.
Katoh Y, Vasudevamurthy G, Nozawa T, Snead LL. Properties of zirconium carbide for nuclear fuel applications. J Nucl Mater. 2013;441(1–3):718.
McGuiness PJČM, Nemanič V, Zajec B, Rečnik A. Hydrogen permeation through TiAlN-coated Eurofer ‘97 steel. Surf Coat Technol. 2011;205(8–9):2709.
Zhang K, Hatano Y. Sealing of pores in sol–gel-derived tritium permeation barrier coating by electrochemical technique. J Nucl Mater. 2011;417(1–3):1229.
Choudhuri G, Neogy S, Sen D, Mazumder S, Srivastava D, Dey GK, Shah BK. Precipitation and growth study of intermetallics and their effect on oxidation behavior in Zr–Sn–Fe–Cr alloy. J Nucl Mater. 2012;430(1–3):205.
Yao Z, Suzuki A, Levchuk D, Chikada T, Tanaka T, Muroga T, Terai T. Hydrogen permeation through steel coated with erbium oxide by sol–gel method. J Nucl Mater. 2009;386–388:700.
Chikada T, Suzuki A, Kobayashi T, Maier H, Terai T, Muroga T. Microstructure change and deuterium permeation behavior of erbium oxide coating. J Nucl Mater. 2011;417(1–3):1241.
Ruppi S, Deposition AL. Microstructure and properties of texture-controlled CVD α-Al2O3 coatings. Int J Refract Met Hard Mater. 2005;23(4–6):306.
Kim SJ, Kim KY. Electrochemical hydrogen permeation measurement through high-strength steel under uniaxial tensile stress in plastic range. Scripta Mater. 2012;66(12):1069.
Zhang Y, Bai KF, Fu ZY, Zhang CL, Zhou H, Wang LG, Zhu SJ, Guan SK, Li DS, Hu JH. Composite coating prepared by micro-arc oxidation followed by sol-gel process and in vitro degradation properties. Appl Surf Sci. 2012;258(7):2939.
Shoaei-Rad V, Bayati MR, Zargar HR, Javadpour J, Golestani-Fard F. In situ growth of ZrO2-Al2O3 nano-crystalline ceramic coatings via micro arc oxidation of aluminum substrates. Mater Res Bull. 2012;47(6):1494.
Yan Y, Han Y, Li D, Huang J, Lian Q. Effect of NaAlO2 concentrations on microstructure and corrosion resistance of Al2O3/ZrO2 coatings formed on zirconium by micro-arc oxidation. Appl Surf Sci. 2010;256(21):6359.
Kung KC, Lee TM, Chen JL, Lui TS. Characteristics and biological responses of novel coatings containing strontium by micro-arc oxidation. Surf Coat Technol. 2010;205(6):1714.
Jung YC, Shin KR, Ko YG, Shin DH. Surface characteristics and biological response of titanium oxide layer formed via micro-arc oxidation in K3PO4 and Na3PO4 electrolytes. J Alloys Compd. 2013;586(S1):S548.
Chai LY, Yu X, Yang ZH, Wang YY, Okido M. Anodizing of magnesium alloy AZ31 in alkaline solutions with silicate under continuous sparking. Corros Sci. 2008;50(12):3274.
Ghasemi VSR, Blawert C, Dietzel W, Kainer KU. The role of anions in the formation and corrosion resistance of the plasma electrolytic oxidation coatings. Surf Coat Technol. 2010;204(9–10):1469.
Wilber S, Thanh NT, Fanor M. Electronic characterization and reactivity of bimetallic clusters of the Ti(Mg)n type for hydrogen storage applications. J Alloys Compd. 2011;509(34):8501.
Zhang SF, Xiang JH, Zhang LH, Zhang YQ, Guo SB. Influence of sodium silicate concentration on properties of micro arc oxidation coatings formed on AZ91HP magnesium alloys. Surf Coat Technol. 2012;206(24):5072.
Demirci EE, Arslan E, Ezirmik KV, Baran Ö, Totik Y, Efeoglu İ. Investigation of wear, corrosion and tribocorrosion properties of AZ91 Mg alloy coated by micro arc oxidation process in the different electrolyte solutions. Thin Solid Films. 2013;528:116.
Zajec B, Nemanič V, Žumer M, Porosnicu C, Lungu CP. Hydrogen permeability through beryllium films and the impact of surface oxides. J Nucl Mater. 2013;443(1–3):185.
Chang LM. Growth regularity of ceramic coating on magnesium alloy by plasma electrolytic oxidation. J Alloys Compd. 2009;468(1–2):462.
Zhao LC, Cui CX, Wang QZ, Bu SJ. Growth characteristics and corrosion resistance of micro-arc oxidation coating on pure magnesium for biomedical applications. Corros Sci. 2010;52(7):2228.
Xu JL, Zhong ZC, Yu DZ, Liu F, Luo JM. Effect of micro-arc oxidation surface modification on the properties of the NiTi shape memory alloy. J Mater Sci Mater Med. 2012;23(12):2839.
Liu YJ, Xu JY, Lin W, Gao C, Zhang JC, Chen XH. Influences of additive on the formation and corrosion resistance of micro-arc oxidation ceramic coatings on aluminum alloy. Rev Adv Mater Sci. 2013;33(2).
Yan YY, Han Y, Li DC, Huang JJ, Lian Q. Effect of NaAlO2 concentrations on microstructure and corrosion resistance of Al2O3/ZrO2 coatings formed on zirconium by micro-arc oxidation. Appl Surf Sci. 2010;256(21):6359.
Samanipour F, Bayati MR, Zargar HR, Golestani-Fard F, Troczynski T, Taheri M. Electrophoretic enhanced micro arc oxidation of ZrO2–HAp–TiO2 nanostructured porous layers. J Alloys Compd. 2011;509(38):9351.
Moya JS, Diaz M, Bartolome JF, Roman E, Sacedon JL, Izquierdo J. Zirconium oxide film formation on zircaloy by water corrosion. Acta Mater. 2000;48(18–19):4749.
Acknowledgments
The project was financially supported by the National Natural Science Foundation (Nos. 51164023 and 513640236), the Program for Young Talents of Science and Technology in Universities of Inner Mongolia Autonomous Region (No. NJYT-13-B10) and the Program for New Century Excellent Talents in University (No. NCET-13-0847).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, ZG., Chen, WD., Yan, SF. et al. Characterization of ZrO2 ceramic coatings on ZrH1.8 prepared in different electrolytes by micro-arc oxidation. Rare Met. 41, 1043–1050 (2022). https://doi.org/10.1007/s12598-015-0503-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-015-0503-8