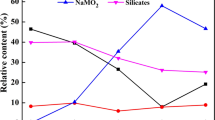
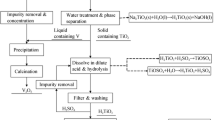
Abstract
By means of energy-dispersive X-ray spectroscopy (EDX) and scanning electron microscope (SEM) analysis, the phase structure characteristics of high titanium slag were analyzed. Through the single factor and the orthogonal experiment methods, the effects of material particle size, mass ratio of acid to ore, roasting temperature, and roasting time on the acidolysis ratio of TiO2 during the process of roasting high titanium slag with concentrated sulfuric acid were systematically investigated. The results show that the sequence of each factor affecting the acidolysis ratio of TiO2 is: mass ratio of acid to ore, roasting time, and roasting temperature. The optimum technological conditions are obtained as mass ratio of acid to ore of 2.1, roasting temperature of 310 °C, roasting time of 75 min, and material particle size of 45–53 µm. The acidolysis ratio of TiO2 is over 96 % under the optimum conditions. The roasting process is proved to be significant in the exploitation and utilization of high titanium slag. The advantages of the proposed roasting process are of high efficiency, low power consumption, and minimum pollution.








Similar content being viewed by others
References
Liu XH, Sui ZT. A new method for preparing rich TiO2 materials from Ti-bearing slag. Univ Technol. 2005;24(4):268.
Zhang SL. Industrial experiment of producing titanium white by acid dissolved titanium slag. Iron Steel Vanadium Titan. 2005;19(3):33.
Dong BH, Wu F, Alajmi Z, Zhang C, Fu T, Ge Y. Sol–gel derived Ta-containing TiO2 films on surface roughened NiTi alloy. Rare Met. 2014;33(1):21.
Hua YX. Metallurgy Process Kinetics Introduction. Beijing: Metallurgical Industry Press; 2004. 9.
Zhang WS, Zhu ZW, Cheng CY. A literature review of titanium metallurgical processes. Hydrometallurgy. 2011;108(3):177.
Jing JL, Zhang QZ, Qiu LY, Liang B. An investigation on the liquid phase digestion of ilmenite in sulfate process TiO2 pigment production. Chem React Eng Technol. 2003;19(4):337.
Wu L, Li XH, Wang ZX, Wang XJ, Li LJ, Fang J, Wu FX, Guo HJ. Preparation of synthetic rutile and metal-doped LiFePO4 from ilmenite. Powder Technol. 2010;199(3):293.
Li C, Liang B, Song H, Xu JQ, Wang XQ. Preparation of porous rutile titania from ilmenite by mechanical activation and subsequent sulfuric acid leaching. Microporous Mesoporous Mater. 2008;115(3):293.
Akhgar BN, Pazouki M, Ranibar M, Hosseinnia A, Keyanpour RM. Preparation of nanosized synthetic rutile from ilmenite concentrate. Miner Eng. 2010;23(7):587.
Li L. Study on acid hydrolysis process of deep reduction slag of Panzhihua V-Ti magnetite. Inorg Chemi Ind. 2010;42(6):52.
Mahmoud MHH, Afifi AAI, Ibrahim IA. Reductive leaching of ilmenite ore in hydrochloric acid for preparation of synthetic rutile. Hydrometallurgy. 2004;73(1):99.
Li C, Liang B, Wang HY. Preparation of synthetic rutile by hydrochloric acid leaching of mechanically activated Panzhihua ilmenite. Hydrometallurgy. 2008;91(2):121.
Liu XH, Sui ZT. Leaching of Ti bearing blast furnace slag by pressuring. Chin J Nonferrous Met. 2002;12(6):1281.
Hou QL, Duan HT, Yang S, Liu YJ, Chen JH. Process of rutile titanium dioxide coated with ZrO2. Chin J Rare Met. 2013;37(3):411.
Jia QY, Que WX, Qiu XK, Zhong P, Chen Jin. Preparation of hierarchical TiO2 microspheres for enhancing photocurrent of dye sensitized solar cells. Sci China-Phys Mech Astron, 2012, 55(7): 1158.
Han YF, Li J, Wang LN, Qu JK. Preparation of titanium dioxide from titania-rich slag by molten NaOH method, International Journal of Minerals. Metall Mater. 2012;19(3):205.
Wang B, Cheng XZ, Han KX, Qin XH, Ma Y. Research of acidolysis performance of acid soluble titanium slag. Iron Steel Vanadium Titan. 2009;30(2):6.
Zhang YJ, Qi T, Zhang Y. A novel preparation of titanium dioxide from titanium slag. Hydrometallurgy. 2009;96(1):52.
Yan CH, Xie ZY, Wang ZF, Zhang ZJ, Wu YY, Zhang M. Comprehensive recovery of low grade tungsten-titanium polymetallic ore in Hubei. Chin J Rare Met. 2013;37(4):650.
Acknowledgments
This project was financially supported by the National Natural Science Foundation of China (Nos. 61372195 and 61304069) and the National Basic Research Program of China (No. 2007CB613603).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sui, LL., Zhai, YC. & Miao, LH. Recovery of titania from high titanium slag by roasting method using concentrated sulfuric acid. Rare Met. 34, 895–900 (2015). https://doi.org/10.1007/s12598-014-0359-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-014-0359-3