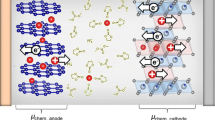
Abstract
A LiFePO4-type lithium secondary battery cell of 8 Ah capacity with a high energy density and power density was developed for hybrid electric vehicle (HEV) applications by optimizing the key raw materials and process design. The 8 Ah class LiFePO4 cell with an energy density of 77.2 Wh·kg−1 exhibits a power density of 2818 W·kg−1 at 50 % SOC (state of charge). The battery shows good cyclic capability with the capacity retention of 81.1 % after 1,870 cycles at 5C charge and 10C discharge rates. It is demonstrated that the cells have an excellent balance of high-power, high-energy, low temperature, and long-life performance for meeting the requirements of HEV.








Similar content being viewed by others
References
Schiermeier, Tollefson J, Scully T, Witze A, Morton O. Titre du document/document title. Nature. 2008;454:816.
Wu Y, Pei F, Jia LL, Liu XL, Zhang WH, Liu P. Overview of recovery technique of valuable metals from spent lithium ion batteries. Chin J Rare Met. 2013;37(2):320.
Bi J, Shao S, Guan W, Wang L. State of charge estimation of Li-ion batteries in electric vehicle based on radial-basis-function neural network. Chin Phys B. 2012;21(11):118801.
Zhao L, Pan HL, Hu YS, Li H, Chen LQ. Spinel lithium titanate (Li4Ti5O12) as novel anode material for room-temperature sodium-ion battery. Chin Phys B. 2012;21(2):028201.
Jaiswal A, Horne CR, Chang O, Zhang W, Kong W, Wang E, Chern T, Doeff MM. Nanoscale LiFePO4 and Li4Ti5O12 for high rate Li-ion batteries. J Electrochem Soc. 2009;156:A1041.
Zaghib K, Dontigny M, Cuerfi A, Charest P, Rodrigues I, Mauger A, Julien CM. Safe and fast-charging Li-ion battery with long shelf life for power applications. J Power Sources. 2011;196(8):3949.
Padhi AK, Nanjundaswamy KS, Goodenough JB. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J Electrochem Soc. 1997;144(4):1188.
Prosini PP, Lisi M, Zane D, Pasquali M. Determination of the chemical diffusion coefficient of lithium in LiFePO4. Solid State Ionics. 2002;148(1–2):45.
Dominko R, Bele M, Gaberscek M, Remskar M, Hanzel D, Pejovnik S, Jamnik J. Impact of the carbon coating thickness on the electrochemical performance of LiFePO4/C composites. J Electrochem Soc. 2005;152(3):A607.
Ravet N, Chouinard Y, Magnan JF, Besner S, Gauthier M, Armand M. Electroactivity of natural and synthetic triphylite. J Power Sources. 2001;97–98:503.
Dominko R, Bele M, Gaberscek M, Remskar M, Hanzel D, Goupil JM, Pejovnik S, Jamnik J. Porous olivine composites synthesized by sol–gel technique. J Power Sources. 2006;153(2):274.
Delacourt C, Poizot P, Levasseur S, Masquelier C. Size effects on carbon-free LiFePO4 powders. Electrochem Solid-State Lett. 2006;9(7):A352.
Nan CY, Lu J, Chen C, Peng Q, Li YD. Solvothermal synthesis of lithium iron phosphate nanoplates. J Mater Chem. 2011;21(27):9994.
Kong F, Kostecki R, Nadeau G, Song X, Zaghib K, Kinoshita K, McLarnon F. In situ studies of SEI formation. J Power Sources. 2001;97–98:58.
Zheng T, Reimers JN, Dahn JR. Effect of turbostratic disorder in graphite carbon hosts on the intercalation of lithium. Phys Rev. 1995;B51(2):734.
Aurbach D, Markovsky B, Shechter A, Ein-Eli Y, Cohen H. A comparative study of synthetic graphite and Li electrodes in electrolyte solutions based on ethylene carbonate-dimethyl carbonate mixtures. J Electrochem Soc. 1996;143(12):3809.
Aurbach D, Gamolsky K, Markovsky B, Gofer Y, Schmidt M, Heider U. On the use of vinylene carbonate (VC) as an additive to electrolyte solutions for Li-ion batteries. Electrochem Acta. 2002;47(9):1423.
Zhang SSXuK, Allen JL, Jow TR. Effect of propylene carbonate oil on the low temperature performance of Li-ion cells. J Power Sources. 2002;110:216.
Smart MC, Ratnakumar BV, Surampudi S, Wang Y, Zhang X, Greenbaum SG, Hightower A, Ahn CC, Fultz B. Irreversible capacities of graphite in low-temperature electrolytes for lithium-ion batteries. J Electrochem Soc. 1999;146(11):3963.
Acknowledgments
This study was financially supported by the State Basic Research Development Program of China (No. 2009CB220100), the Ministry of Science and Technology (MOST) of China, US-China Collaboration on Cutting-Edge Technology Development of Electric Vehicles(No.2010DFA72760), the National Natural Science Foundation of China (Nos. 50901009 and 51271029), and the Fundamental Research Funds for the Central Universities (No. 12QNJJ013).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Deng, LZ., Wu, F., Gao, XG. et al. Development of a LiFePO4-based high power lithium secondary battery for HEVs applications. Rare Met. 39, 1457–1463 (2020). https://doi.org/10.1007/s12598-014-0316-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-014-0316-1