Abstract
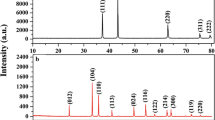
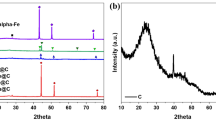
A series of Fe2O3/Al2O3, Fe2O3/CeO2, Ce0.7Zr0.3O2, and Fe2O3/Ce1−x Zr x O2 (x = 0.1–0.4) oxides was prepared and their physicochemical features were investigated by X-ray diffraction (XRD), transmission electron microscope (TEM), and H2-temperature-programmed reduction (H2-TPR) techniques. The gas–solid reactions between these oxides and methane for syngas generation as well as the catalytic performance for selective oxidation of carbon deposition in O2-enriched atmosphere were investigated in detail. The results show that the samples with the presence of Fe2O3 show much higher activity for methane oxidation compared with the Ce0.7Zr0.3O2 solid solution, while the CeO2-contained samples represent higher CO selectively in methane oxidation than the Fe2O3/Al2O3 sample. This suggests that the iron species should be the active sites for methane activation, and the cerium oxides provide the oxygen source for the selective oxidation of the activated methane to syngas during the reaction between methane and Fe2O3/Ce0.7Zr0.3O2. For the oxidation process of the carbon deposition, the CeO2-containing samples show much higher CO selectivity than the Fe2O3/Al2O3 sample, which indicates that the cerium species should play a very important role in catalyzing the carbon selective oxidation to CO. The presence of the Ce–Zr–O solid solution could induce the growth direction of the carbon filament, resulting in a loose contact between the carbon filament and the catalyst. This results in abundant exposed active sites for catalyzing carbon oxidation, strongly improving the oxidation rate of the carbon deposition over this sample. In addition, the Fe2O3/Ce0.7Zr0.3O2 also represents much higher selectivity (ca. 97 %) for the conversion of carbon to CO than the Fe2O3/CeO2 sample, which can be attributed to the higher concentration of reduced cerium sites on this sample. The increase of the Zr content in the Fe2O3/Ce1−x Zr x O2 samples could improve the reactivity of the materials for methane oxidation, but it also reduces the selectivity for CO formation.








Similar content being viewed by others
References
Thursfield A, Murugan A, Franca R, Metcalfe IS. Chemical looping and oxygen permeable ceramic membranes for hydrogen production-a review. Energy Environ Sci. 2012;5(6):7421.
Li KZ, Wang H, Wei YG, Ao XQ, Liu MC. Partial oxidation of methane to synthesis gas using lattice oxygen. Prog Chem. 2009;20(9):1306.
Pantu P, Kim K, Gavalas GR. Methane partial oxidation on Pt/CeO2–ZrO2 in the absence of gaseous oxygen. Appl Catal A. 2000;193(1–2):203.
Dai XP, Li RJ, Yu CC, Hao ZP. Unsteady-state direct partial oxidation of methane to synthesis gas in a fixed-bed reactor using AFeO3 (A = La, Nd, Eu) perovskite-type oxides as oxygen storage. J Phys Chem B. 2006;110(45):22525.
Takenaka S, Tomikubo Y, Kato E, Otsuka K. Sequential production of H2 and CO over supported Ni catalysts. Fuel. 2004;83(1):47.
Zhang T, Amiridis MD. Hydrogen production via the direct cracking of methane over silica-supported nickel catalysts. Appl Catal A. 1998;167(2):161.
Aiello R, Fiscus JE, Zur Loye HC, Amiridis MD. Hydrogen production via the direct cracking of methane over Ni/SiO2 catalyst deactivation and regeneration. Appl Catal A. 2004;192(2):227.
Otsuka K, Takenaka S, Ohtsuki H. Production of pure hydrogen by cyclic decomposition of methane and oxidative elimination of carbon nanofibers on supported-Ni-based catalysts. Appl Catal A. 2004;273(1–2):113.
Li J, Smith KJ. Methane decomposition and catalyst regeneration in a cyclic mode over supported Co and Ni catalysts. Appl Catal A. 2008;349(1–2):116.
Odier E, Schuurman Y, Mirodatos C. Non-stationary catalytic cracking of methane over ceria-based catalysts: mechanistic approach and catalyst optimization. Catal Today. 2007;127(1–4):230.
Fathi M, Bjorgum E, Viig Tm Rokstad OA. Partial oxidation of methane to synthesis gas: elimination of gas phase oxygen. Catal Today. 2000;63(2–4):489.
Dai XP, Wu Q, Li RJ, Yu CC, Hao ZP. Hydrogen production from a combination of the water-gas shift and redox cycle process of methane partial oxidation via lattice oxygen over LaFeO3 perovskite catalyst. J Phys Chem B. 2006;110(51):25856.
Li KZ, Wang H, Wei YG, Yan DX. Selective oxidation of carbon using iron-modified cerium oxide. J Phys Chem C. 2009;113(34):15288.
Gu ZH, Li KZ, Wang H, Wei YG. Syngas production from methane over CeO2–Fe2O3 mixed oxides using a chemical-looping method. Kinet Catal. 2013;54(3):340.
Atribak I, Azambre B, Bueno-Lopez A, Garcia–Garcia A. NO x adsorption/desorption processes over Ce0.76Zr0.24O2 and their influence on Desoot activity: effect of the catalyst calcination temperature. Top Catal. 2009;52(13–20):2092.
Magnacca G, Cerrato G, Morterra C, Signoretto M, Somma F, Pinna F. Structural and surface characterization of pure and sulfated iron oxides. Chem Mater. 2003;15(3):675.
Atribak I, Buenolopez A, Garciagarcia A. Combined removal of diesel soot particulates and NO x over CeO2–ZrO2 mixed oxides. J Catal. 2008;259(1):123.
Qiao D, Lu G, Liu X, Guo Y, Wang Y, Guo Y. Preparation of Ce1−x Fe x O2 solid solution and its catalytic performance for oxidation of CH4 and CO. J Mater Sci. 2011;46(10):3500.
Laguna OH, Centeno MA, Boutonnet M. Fe-doped ceria solids synthesized by the microemulsion method for cooxidation reactions. Appl Catal B. 2011;106(3–4):621.
Li KZ, Wang H, Wei Y, Yan DX. Transformation of methane into synthesis gas using the redox property of Ce–Fe mixed oxides: effect of calcination temperature. Int J Hydrogen Energy. 2011;36(5):3471.
Li KZ, Haneda M, Ozawa M. Enhancement of reducibility and oxygen storage capacity (OSC of Ce–Fe mixed oxides by repetitive redox treatment. Chem Lett. 2012;41(9):837.
Sadykov VA, Kumetsova TG, Alikina GM. Ceria-based fluorite-like oxide solid solutions as catalysts of methane selective oxidation into syngas by the lattice oxygen: synthesis, characterization and performance. Catal Today. 2004;93–95(SI):45.
Hu ZY, Yang Y, Sun B, Zhang P, Wang WC, Shao XH. Dissociations of O2 molecules on ultrathin Pb(111) films: first-principles plane wave calculations. Chin Phys B. 2012;21(1):016801.
Wei YG, Wang H, Li KZ, Zhu X, Du YP. Preparation and characterization of Ce1−x Ni x O2 as oxygen carrier for selective oxidation methane to syngas in absence of gaseous oxygen. J Rare Earths. 2010;28(SI):357.
Li KZ, Wang H, Wei YG, Yan DX. Direct conversion of methane to synthesis gas using lattice oxygen of CeO2–Fe2O3 complex oxides. Chem Eng J. 2010;156(3):512.
Li KZ, Wang H, Wei YG, Yan DX. Syngas production from methane and air via a redox process using Ce–Fe mixed oxides as oxygen carriers. Appl Catal B. 2010;97(3–4):361.
Li KZ, Wang H, Wei YG, Yan DX. Partial oxidation of methane to syngas with air by lattice oxygen transfer over ZrO2-modified Ce–Fe mixed oxides. Chem Eng J. 2011;173(2):574.
Takenaka S, Nomura K, Hanaizumi N. Storage and formation of pure hydrogen mediated by the redox of modified iron oxides. Appl Catal A. 2005;282(1–2):333.
Gemmi M, Merlini M, Cornaro U, Ghisletti D, Artioli G. In situ simultaneous synchrotron powder diffraction and mass spectrometry study of methane anaerobic combustion on iron-oxide-based oxygen carrier. J Appl Crystallogr. 2005;38:353.
Otsuka K, Wang Y, Sunada E, Yamanaka I. Direct partial oxidation of methane to synthesis gas by cerium oxide. J Catal. 1998;175(2):152.
Otsuka K, Wang Y, Nakamura M. Direct conversion of methane to synthesis gas through gas-solid reaction using CeO2–ZrO2 solid solution at moderate temperature. Appl Catal A. 1999;183(2):317.
Takenaka S, Serizawa M, Otsuka K. Formation of filamentous carbons over supported Fe catalysts through methane decomposition. J Catal. 2004;222(2):520.
Cheng JS, Wang QL, Wang H, Dar YM. Preparation and catalytic activity of CO-resistant catalyst core-shell Au@Pt/C for methanol oxidation. Rare Met. 2012;31(5):446.
Kašpar J, Fornasiero P, Graziani M. Use of CeO2-based oxides in the three-way catalysis. Catal Today. 1999;50(2):285.
Rocchini E, Trovarelli A, Llorca J, Graham GW. Relationships between structural/morphological modifications and oxygen storage-redox behavior of silica-dope ceria. J Catal. 2000;194(2):461.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (Nos. 51004060, 51104074, and 51174105) and the Natural Science Foundation of Yunnan Province (No. 2010ZC018).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sang, XL., Li, KZ., Wang, H. et al. Selective oxidation of methane and carbon deposition over Fe2O3/Ce1−x Zr x O2 oxides. Rare Met. 33, 230–238 (2014). https://doi.org/10.1007/s12598-013-0173-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-013-0173-3