Abstract
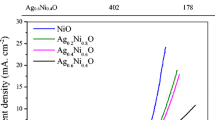
From an aqueous mixture of Ag(I)-EDTA complex and Ni(II) nitrate, silver and nickel particles were co-deposited on the surface of titanium substrates by the hydrothermal method using hydrazine hydrate as a reduction agent. The prepared titanium-supported nano-scale Ag and Ag-Ni particles (nano Ag/Ti, nano Ag86Ni14/Ti, nano Ag77Ni23/Ti, and nano Ag74Ni26/Ti) exhibit nanoporous 3D network textures. Their electrocatalytic activity towards hydrazine oxidation in alkaline solutions was evaluated by cyclic voltammetry and chronoamperometry. The results show that the four samples present a low onset potential of ca. −0.60 V vs. SCE and considerably high and stable anodic current densities for hydrazine oxidation. Among them, the nano Ag86Ni14/Ti electrode exhibits the highest anodic current density towards hydrazine oxidation, showing an increment of electro-active sites on the nano Ag86Ni14/Ti due to the addition of Ni to Ag particles.
Similar content being viewed by others
References
Yamada K., Asazawa K., Yasuda K., Ioroib T., Tanaka H., Miyazaki Y., and Kobayashi T., Investigation of PEM type direct hydrazine fuel cell, J. Power Sources, 2003, 115: 236.
Razmi-Nerbin H. and Pournaghi-Azar M.H., Nickel pentacyanonitrosylferrate film modified aluminum electrode for electrocatalytic oxidation of hydrazine, J. Solid State Electrochem., 2002, 6: 126.
Rosca V. and Koper M.T.M., Electrocatalytic oxidation of hydrazine on platinum electrodes in alkaline solutions, Electrochim. Acta, 2008, 53: 5199.
Li N., Tianyan Y., Gui J.Y., Erkang W., and Shaojun D., Electrocatalytic oxidation of hydrazines at a 4-pyridyl hydroquinone self-assembled platinum electrode and its application to amperometric detection in capillary electrophoresis, J. Electroanal. Chem., 1998, 448: 79.
García Azorero M.D., Marcos M.L., and González Velasco J., Influence of changes in the total surface area and in the crystalline surface composition of Pt electrodes on their electrocatalytic properties with respect to the electro-oxidation of hydrazine, Electrochim. Acta, 1994, 39: 1909.
Gómez R., Orts J.M., Rodes A., Feliu J.M., and Aldaz A., The electrochemistry of nitrogen-containing compounds at platinum single crystal electrodes: Part 1. Hydrazine behaviour on platinum basal planes in sulphuric acid solutions, J. Electroanal. Chem., 1993, 358: 287.
Kodera T. and Honda Kita M.H., Electrochemical behaviour of hydrazine on platinum in alkaline solution, Electrochim. Acta, 1985, 30: 669.
Kokkinidis G. and Jannakoudakis P.D., Influence of the electrosorption of heavy metals on hydrazine oxidation on platinum, J. Electroanal. Chem., 1981, 130: 153.
Perek M. and Bruckenstein S., An isotopic labeling investigation of the mechanism of the electrooxidation of hydrazine at platinum: An electrochemical mass spectrometric study, J. Electroanal. Chem., 1973, 47: 329.
Harrison J.A. and Khan Z.A., The oxidation of hydrazine on platinum in acid solution, J. Electroanal. Chem., 1970, 28: 131.
Kořínek K., Koryta J., and Musilová M., Electro-oxidation of hydrazine on mercury, silver and gold electrodes in alkaline solutions, J. Electroanal. Chem., 1969, 21: 319.
Salimi A. and Hallaj R., Adsorption and Reactivity of chlorogenic acid at a hydrophobic carbon ceramic composite electrode: Application for the amperometric detection of hydrazine, Electroanalysis, 2004, 16: 1964.
Salimi A. and Abdi K., Enhancement of the analytical properties and catalytic activity of a nickel hexacyanoferrate modified carbon ceramic electrode prepared by two-step sol-gel technique: Application to amperometric detection of hydrazine and hydroxylamine, Talanta, 2004, 63: 475.
Pinter J.S., Brown K.L., DeYoung P.A., and Peaslee G.F., Amperometric detection of hydrazine by cyclic voltammetry and flow injection analysis using ruthenium modified glassy carbon electrodes, Talanta, 2007, 71: 1219.
Batchelor-McAuley C., Banks C.E., Simm A.O., Jones T.G.J., and Compton R.G., The electroanalytical detection of hydrazine: A comparison of the use of palladium nanoparticles supported on boron-doped diamond and palladium plated BDD microdisc array, Analyst, 2006, 131: 106.
Méndez M.A., Súarez M.F., Cortés M.T., and Sarria V.M., Electrochemical properties and electro-aggregation of silver carbonate sol on polycrystalline platinum electrode and its electrocatalytic activity towards glyphosate oxidation, Electrochem. Commun., 2007, 9: 2585.
Ben Aoun S., Sook Bang G., Koga T., Nonaka Y., Sotomura T., and Taniguchi I., Electrocatalytic oxidation of sugars on silver-UPD single crystal gold electrodes in alkaline solutions, Electrochem. Commun., 2003, 5: 317.
Ni K., Chen L., and Lu G.X., Synthesis of silver nanowires with different aspect ratios as alcohol-tolerant catalysts for oxygen electroreduction, Electrochem. Commun., 2008, 10: 1027.
Sleightholme A.E.S., Varcoe J.R., and Kucernak A.R., Oxygen reduction at the silver/hydroxide-exchange membrane interface, Electrochem. Commun., 2008, 10: 151.
Kongkanand A. and Kuwabata S., Oxygen reduction at silver monolayer islands deposited on gold substrate, Electrochem. Commun., 2003, 5: 133.
Gao G.Y., Guo D.J., Wang C., and Li H.L., Electrocrystallized Ag nanoparticle on functional multi-walled carbon nanotube surfaces for hydrazine oxidation, Electrochem. Commun., 2007, 9: 1582.
Yang G.W., Gao G.Y., Wang C., Xu C.L., and Li H.L., Controllable deposition of Ag nanoparticles on carbon nanotubes as a catalyst for hydrazine oxidation, Carbon, 2008, 46: 747.
Yi Q.F., Huang W., Zhang J.J., Liu X.P., and Li L., A novel titanium-supported nickel electrocatalyst for cyclohexanol oxidation in alkaline solutions, J. Electroanal. Chem., 2007, 610: 163.
Yi Q.F., Chen A., Huang W., Zhang J.J., Liu X.P., Xu G.R., and Zhou Z.H., Titanium-supported nanoporous bimetallic Pt-Ir electrocatalysts for formic acid oxidation, Electrochem. Commun., 2007, 9: 1513.
Koczkur K., Yi Q.F., and Chen A., Nanoporous Pt-Ru networks and their electrocatalytical properties, Adv. Mater., 2007, 19: 2648.
Droog J.M.M., Alderliesten P.T., and Bootsma G.A., Initial stages of anodic oxidation of silver in sodium hydroxide solution studied by potential sweep voltammetry and ellipsometry, J. Electroanal. Chem., 1979, 99: 173.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yi, Q., Li, L., Yu, W. et al. Novel nanoporous binary Ag-Ni electrocatalysts for hydrazine oxidation. Rare Metals 29, 26–31 (2010). https://doi.org/10.1007/s12598-010-0005-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-010-0005-7