Abstract
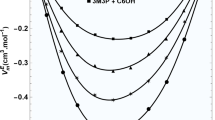
The nature of hydrogen bonding in methanol solutions of Policarbinol was investigated by the methods of Viscometry and Light scattering. The intrinsic viscosity (η) of narrow fraction of Policarbinol in methanol in presence of various amounts of hydrogen bond acceptor—sodium hydroxide (NaOH) was determined using Viscometry. It was established that the values of with addition of are passing through the extreme values due to the fact that intermolecular hydrogen bonds are broken first resulting in the decrease of, and then break in intramolecular hydrogen bonds is leading to increase of . Huggins constant reaches maximum value at complete suppression of intermolecular hydrogen bonds. Results of Light scattering show that the addition of an acceptor hydrogen bonds decreases the mean-square radii of gyration symbatically to values. The most compact size of macromolecules corresponds to minimum of values.



Similar content being viewed by others
References
Missopolinou D, Panayiotou C (1999) Hydrogen-bonding cooperativity and competing inter- and intramolecular associations: a unified approach. J Phys Chem A 102:3574–3581
Missopolinou D, Panayiotou C (1999) On intermolecular and intramolecular hydrogen bonding. Fluid Phase Equilib 156:51–56
Crupi V, Majolino D, Migliardo P, Venuti V (2000) Inter- and intramolecular hydrogen bond in liquid polymers: a Fourier transform infrared response. Mol Phys 98:1589–1594
Huggins MT, Lightner DA (2001) Intramolecular hydrogen bonding between remote termini. Tetrahedron 57(12):2279–2287
Boiadjiev SE, Lightner DA (1999) Intramolecular hydrogen bonding and its influence on conformation. Circular dichroism of chiral bilirubin analogs. Tetrahedron Asymmetry 10(13):2535–2550
Panayiotou C, Pantoula M, Stefanis E, Tsivintzelis I, Economou IG (2004) Nonrandom hydrogen-bonding model of fluids and their mixtures. Pure fluids. Ind Eng Chem Res 43(20):6592–6606
Bilton C, Allen FH, Shields GP, Howard AK (2000) Intramolecular hydrogen bonds: common motifs, probabilities of formation and implications for supramolecular organization. Acta Crystallogr B Struct Sci 56(5):849–856
Garbuz NI, Solovei LP, Kovganko NV, Chernov Yu G (2002) Intramolecular hydrogen bond in steroid 5-Hydroxy-6-Ketones and 5-Hydroxy-6-Ketoximes of the stigmastane series. J Appl Spectrosc 69(1):1573–1864
Albrecht M, Witt K, Fröhlich R, Kataeva O (2002) Inter- and intramolecular hydrogen bonding in amide- and urea-substituted 8-hydroxyquinoline derivatives. Tetrahedron 58(3):561–567
Yamamoto T, Oguro D, Kubota K (1979) Viscometric and light scattering analyses of CHCl3 solutions of Poly(3-alkylthiophene-2,5-diyl)s. Biochim Biophys Acta (BBA) - Gen Subj 586:179–188
Liu M, Cheng R, Wu C, Qian R (1997) Viscometric investigation of intramolecular hydrogen bonding cohesional entanglement in extremely dilute aqueous solution of poly vinyl alcohol. J Polym Sci B Polym Phys 35:2421–2427
Lee JH, Lee CS (2006) Intra- and intermolecular hydrogen-bonding model and its application to 2-alkoxyethanol systems. Ind Eng Chem Res 45(5):1817–1821
Duan M, Fang S, Guo H, Zhang L (2009) Viscometric studies of interactions between hydrophobically modified acrylamide copolymer and poly N-isopropylacrylamide) in dilute solutions. J Macromol Sci B 48(4):834–843
Eskin V (1990) Rassejanie sveta rastvorami polimerov i svoistva makromolekul, Khimiya, Moscow. (In Russian)
Danielyan VA, Barkhudaryan VG, Macoyan SG (1969) Arm Chim J 22:774–777, In Russian
Pals DT, Hermans JJ (1950) J Polymer Sci 5:733
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barkhudaryan, V.G. Viscometric and light scattering investigation of the hydrogen bonds in the solutions of policarbinol. Int J Plast Technol 16, 17–23 (2012). https://doi.org/10.1007/s12588-012-9029-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12588-012-9029-1