Abstract
It is now evident that exercise training leads to increases in monocarboxylate transporter (MCT)1 and MCT4, but little is known about the mechanisms of coupling muscle contraction with these changes. The aim of this study was to investigate the effect of 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside (AICAR) induced activation of AMP-activated protein kinase (AMPK) on MCT1, MCT4, and GLUT4 in denervated muscle. Protein levels of MCT4 and GLUT4 after 10 days of denervation were significantly decreased in mice gastrocnemius muscle, while MCT1 protein levels were not altered. AICAR treatment for 10 days significantly increased MCT4, and GLUT4 protein levels in innervated muscle as shown in previous studies. We found that the MCT1 protein level was also increased in AICAR treated innervated muscle. AICAR treatment prevented the decline in MCT4 and GLUT4 protein levels in denervated muscle. Thus, the current study suggests that MCT1 and MCT4 protein expression in muscles, as well as GLUT4, may be regulated by AMPK-mediated signal pathways, and AMPK activation can prevent denervation-induced decline in MCT4 protein.
Similar content being viewed by others
Introduction
Lactate transport across the plasma membrane is facilitated by a family of monocarboxylate transporters (MCTs) [1]. Among the 14 identified MCT isoforms, MCT1 and MCT4 are considered to be the key transporters in skeletal muscle; MCT1 facilitates lactate uptake, while MCT4 facilitates lactate extrusion [2, 3]. Studies have shown that muscle contractile activity appears to be important for the regulation of MCTs in skeletal muscle [4]. Protein contents of MCT1 and MCT4 can be increased by exercise training, and returned to the baseline levels after training cessation [5]. However, the signal pathways involved in changes in protein expression of MCTs remain largely unknown.
AMP-activated protein kinase (AMPK) is known as a master sensor of changes in cellular energy metabolism, and AMPK activation by 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside (AICAR) administration stimulates the expression of proteins involved with carbohydrate metabolism, mimicking effects of exercise training [6, 7]. Skeletal muscle AMPK activity is increased during exercise [8, 9]. Moreover, GLUT4 protein levels, glycogen stores, and the activity of hexokinase and mitochondria oxidative enzymes are all increased in response to both exercise training and chronic AICAR administration [10]. Recently, it has been reported that MCT4 protein content was increased by AICAR treatment in mice [11]. Hence, it is possible that muscle contractile activity dependent changes in lactate transporters may also be dependent on AMPK.
To examine this issue, muscle denervation provides a suitable model. Neural regulation of muscle contraction is eliminated in denervated muscle, while the contralateral muscle from the same animal serves as control; all other conditions can be kept the same. MCT1 and MCT4 proteins have been shown to decrease in denervated muscle [12]. In the light of these previous findings, the aim of this study was to investigate the effect of activation of AMPK on MCT1 and MCT4 with or without immobilization due to denervation. We hypothesized that AMPK activation could rescue the decreases in MCT proteins with muscle inactivity.
Materials and methods
Animals
Eight-week-old male ICR mice (CLEA Japan, Japan) were used. Food (Oriental Yeast, Japan) and water were provided ad libitum. The room temperature was maintained at 23 ± 2 °C with a 12 h light and 12 h dark cycle. All protocols were approved by the Animal Experimental Committee of the University of Tsukuba and were in accordance with the Regulations for Animal Experiments at the University and Fundamental Guidelines for Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Experimental design
Mice were anesthetized with ethyl ether, and the sciatic nerve of the right hindlimb was severed as we previously described [13]. Briefly, a small incision was made in the posterior aspect of the hindlimb to expose the sciatic nerve at the level of the femoral trochanter, and then the exposed nerve portion was cut off using small operating scissors at least 5.0 mm. The skin was closed with surgical glue. The contralateral hindlimb served as control. After the surgery, mice were subcutaneously injected with AICAR (0.5 mg/g body wt) (Toronto Research Chemicals, Toronto, Canada) or saline for 10 days. We selected 10 days because we found that MCT1 and MCT4 proteins were increased more robustly after 10 days of AICAR treatment than after 5 days in preliminary experiments (data not shown). After 24 h from the last injection, mice were sacrificed by cervical dislocation. The gastrocnemius muscle was dissected out quickly from each mouse, and frozen in liquid nitrogen and stored at −80 °C until further analysis. Although it is known that the gastrocnemius muscle can be visually divided into red and white portions as in our previous reports [14, 15], we used the whole gastrocnemius muscle in this study to avoid the possibility of some artificial effects given that the muscle size would be dramatically changed after denervation.
Western blotting
Muscle samples were homogenized and prepared for electrophoresis, followed by Western blotting as we have previously described [16, 17]. Protein concentrations of homogenate samples were measured using a BCA protein assay kit (Pierce, Pittsburgh, PA, USA). Antibodies against MCT1 and MCT4 were raised in rabbits against the oligopeptide corresponding to the C-terminal region of the respective MCT (Qiagen, Japan) and have been used in previous studies [16, 18]. GLUT4 and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) antibodies were obtained from Millipore (Billerica, MA, USA). Total AMPK-α, phospho-AMPK-α (Thr172) antibodies were obtained from Cell Signaling (Danvers, MA, USA). Blots were developed via enhanced chemiluminescence (GE Healthcare, Amersham, UK) and subsequently quantified with the ChemiDoc system (Bio-Rad Laboratories, Hercules, CA, USA). Ponceau S staining was used to control for equal loading and transfer between lanes [19].
Statistical analysis
All data were expressed as mean ± SE. The significance of differences between groups was evaluated by one-way analysis of variance (ANOVA), followed by Bonferroni post hoc tests (GraphPad Prism 5.0, La Jolla, CA, USA). Statistical significance was defined as p < 0.05.
Results
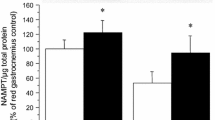
Table 1 summarizes the results of body weight and gastrocnemius muscle weight. The gastrocnemius muscles from saline-treated denervated (SD) and AICAR-treated denervated (AD) groups were significantly atrophied after 10 days compared with muscles of saline-treated control (SC) and AICAR-treated control (AC) groups (Table 1). There was no difference in either body weight or gastrocnemius muscle weight between saline-treated (SC and SD) and AICAR-treated (AC and AD) groups (Table 1). MCT1 (+32 %), MCT4 (+30 %), GLUT4 (+76 %), and PGC-1α (+22 %) protein levels were significantly higher in the AC group compared with the SC group (Fig. 1). Protein levels of MCT4 (−59 %), GLUT4 (−45 %), and PGC-1α (−37 %) were significantly lower in the SD group compared with the SC group, while MCT1 protein level was not altered by 10 days of denervation (Fig. 1). Total AMPK protein level was significantly lower in the SD group compared with the SC group (Fig. 2). There was no difference in phospho-AMPK protein levels between the SC group and all other groups (Fig. 2).
MCT1, MCT4, GLUT4, and PGC-1α protein expression in gastrocnemius muscle in the saline-treated control group (SC) and saline-treated denervated group (SD), AICAR-treated control group (AC), and AICAR-treated denervated group (AD). All values are reported as mean ± SE (n = 6). *Significantly different from SC group (p < 0.05)
Total AMPK and Phosphorylated AMPK (at Thr172) protein expression in gastrocnemius muscle in the saline-treated control group (SC) and saline-treated denervated group (SD), AICAR-treated control group (AC), and AICAR-treated denervated group (AD). All values are reported as mean ± SE (n = 6). *Significantly different from SC group (p < 0.05)
Discussion
Although it is now clear that exercise training leads to increases in MCT1 and MCT4, little is known about the mechanisms of coupling muscle contraction with these changes. AMPK is a possible candidate for regulating exercise-induced changes in MCT expression, because it is known to regulate various proteins related energy metabolism. In the current study, we found that AICAR treatment for 10 days significantly increased MCT1, MCT4, and GLUT4 protein levels in innervated muscle. To our knowledge, this is the first study to report the increase in MCT1 protein by AICAR treatment, while increases in GLUT4 and MCT4 matched previous reports [11, 20]. These results suggested that AMPK activation is important for up-regulating glucose and lactate transporters in skeletal muscle. Previously, Furugen et al. [11] reported that AMPK activation increased MCT4 protein both in white and red gastrocnemius muscles. Therefore, we speculate that there is little effect of fiber types at least in gastrocnemius muscle.
In this study, the levels of GLUT4 and MCT4 proteins were decreased when muscle activity was eliminated by denervation. Moreover, we demonstrated that these declines in GLUT4 and MCT4 proteins could be prevented by AMPK activation. It is reported that AICAR treatment prevented the decrease in GLUT4 and mitochondrial proteins that is typically seen after denervation [20, 21]. Together, these results strongly support the notion that AMPK is important for muscle contractile activity dependent changes in proteins related to energy metabolism, including MCT4. Supporting the importance of AMPK for MCT expression, AICAR-induced MCT4 up-regulation can be blocked by Compound C, an AMPK inhibitor [11]. In the current study, we found that the total AMPK protein level was decreased in denervated muscle, while the phospho-AMPK protein level was not altered. AMPK activity is known to return to basal a few hours after a single AICAR injection [22] or acute exercise [23]. Moreover, exercise training does not alter AMPK activity at rest [24]. Thus, the repeated transient activation of AMPK might be needed to increase or maintain the downstream targets.
At present, the downstream pathways of AMPK for regulating MCT are mostly unknown. As a downstream target of AMPK, PGC-1α is known as a master regulator of genes involved oxidative metabolism [25, 26]. Benton et al. [19] reported a strong relationship between PGC-1α and MCT1 proteins both in chronically stimulated and PGC-1α transfected muscles. We found that AICAR treatment increased PGC-1α protein content both in innervated and denervated muscles. Importantly, it is reported that PGC-1α is required for AICAR-induced up-regulation of GLUT4 [27]. Therefore, AICAR-induced changes in MCT1 may be dependent on PGC-1α. In contrast to PGC-1α, hypoxia inducible factor-1α (HIF-1α) regulates genes involved in glycolytic metabolism, including MCT4 [28, 29]. However, because AICAR treatment does not activate HIF-1α under normoxic conditions [30, 31], AICAR-induced up-regulation of MCT4 may be through a HIF-1α independent mechanism. Another possible mechanism regulating MCT is its substrate concentration, as a previous study has demonstrated that MCT1 expression was increased in L6 muscle cells with high lactate concentrations [32]. Moreover, mitochondrial myopathy patients with lactic acidosis have higher MCT1 and MCT4 protein content, in spite of the apparent decrease in physical activity in daily life [33]. Importantly, it is well known that ACIAR induces an increase in glycolytic flux [34] and the subsequent increase in blood lactate concentration [35, 36]. Further study is needed to investigate molecular mechanisms of regulating MCT expression.
Previous studies reported that MCT1 and MCT4 proteins in rat muscles were decreased after denervation for 3 weeks with concomitant decreases in lactate transport [37, 38], but not after 3 days [12]. In the current study, while we found the decline in MCT4 protein after 10 days of denervation, the MCT1 protein level was not altered. This could imply that the duration of denervation was not enough to down-regulate MCT1. Furthermore, we previously reported that the protein level of MCT1 but not MCT4 could be maintained by moderate-intensity training in well-trained horses [39]. Thus, we speculate that MCT4 might be more sensitive to muscle contractile activity than MCT1. MCT1 is a high-affinity lactate transporter (Km: ~3.5–8.3 mM), and MCT4 is a low-affinity lactate transporter (Km: 25–31 mM) [40]. Our results might suggest that MCT1 is more important in most physiological conditions than MCT4, except when lactate concentration rises to very high levels, such as during intense exercise.
In summary, we have shown that chronic activation of AMPK by AICAR injection increased MCT1, MCT4, and GLUT4 protein levels in innervated muscle, and prevented the decline in MCT4 and GLUT4 protein levels in denervated muscle. These results suggest that MCT1, MCT, and GLUT4 proteins, as well as PGC-1α, may be regulated by AMPK-mediated signal pathways in skeletal muscles, and activation of the AMPK-mediated signal pathways can prevent denervation-induced decline in MCT4 protein. Therefore, it is possible that AMPK activation is one of the key factors in the exercise-induced adaptations of lactate transporters in skeletal muscle. Our data could be beneficial for future clinical application.
References
Bonen A (2000) Lactate transporters (MCT proteins) in heart and skeletal muscles. Med Sci Sports Exerc 32:778–789
Juel C, Halestrap A (1999) Lactate transport in skeletal muscle — role and regulation of the monocarboxylate transporter. J Physiol 517:633–642
Manning Fox JE, Meredith D, Halestrap AP (2000) Characterisation of human monocarboxylate transporter 4 substantiates its role in lactic acid efflux from skeletal muscle. J Physiol 529:285–293
Thomas C, Bishop DJ, Lambert K, Mercier J, Brooks GA (2012) Effects of acute and chronic exercise on sarcolemmal MCT1 and MCT4 contents in human skeletal muscles: current status. Am J Physiol Regul Integr Comp Physiol 302:R1–R14
Burgomaster KA, Cermak NM, Phillips SM, Benton CR, Bonen A, Gibala MJ (2007) Divergent response of metabolite transport proteins in human skeletal muscle after sprint interval training and detraining. Am J Physiol Regul Integr Comp Physiol 292:R1970–R1976
Jorgensen SB, Richter EA, Wojtaszewski JF (2006) Role of AMPK in skeletal muscle metabolic regulation and adaptation in relation to exercise. J Physiol 574:17–31
Kahn BB, Alquier T, Carling D, Hardie DG (2005) AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1:15–25
Nielsen JN, Mustard KJ, Graham DA, Yu H, MacDonald CS, Pilegaard H, Goodyear LJ, Hardie DG, Richter EA, Wojtaszewski JF (2003) 5′-AMP-activated protein kinase activity and subunit expression in exercise-trained human skeletal muscle. J Appl Physiol 94:631–641
Chen ZP, Stephens TJ, Murthy S, Canny BJ, Hargreaves M, Witters LA, Kemp BE, McConell GK (2003) Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes 52:2205–2212
Winder WW (2001) Energy-sensing and signaling by AMP-activated protein kinase in skeletal muscle. J Appl Physiol 91:1017–1028
Furugen A, Kobayashi M, Narumi K, Watanabe M, Otake S, Itagaki S, Iseki K (2011) AMP-activated protein kinase regulates the expression of monocarboxylate transporter 4 in skeletal muscle. Life Sci 88:163–168
Wilson MC, Jackson VN, Heddle C, Price NT, Pilegaard H, Juel C, Bonen A, Montgomery I, Hutter OF, Halestrap AP (1998) Lactic acid efflux from white skeletal muscle is catalyzed by the monocarboxylate transporter isoform MCT3. J Biol Chem 273:15920–15926
Machida M, Takeda K, Yokono H, Ikemune S, Taniguchi Y, Kiyosawa H, Takemasa T (2012) Reduction of ribosome biogenesis with activation of the mTOR pathway in denervated atrophic muscle. J Cell Physiol 227:1569–1576
Enoki T, Yoshida Y, Lally J, Hatta H, Bonen A (2006) Testosterone increases lactate transport, monocarboxylate transporter (MCT) 1 and MCT4 in rat skeletal muscle. J Physiol 577:433–443
Yoshida Y, Holloway G, Ljubicic V, Hatta H, Spriet L, Hood D, Bonen A (2007) Negligible direct lactate oxidation in subsarcolemmal and intermyofibrillar mitochondria obtained from red and white rat skeletal muscle. J Physiol 582:1317–1335
Kitaoka Y, Machida M, Takemasa T, Hatta H (2011) Expression of monocarboxylate transporter (MCT) 1 and MCT4 in overloaded mice plantaris muscle. J Physiol Sci 61:467–472
Ikeda S, Kawamoto H, Kasaoka K, Hitomi Y, Kizaki T, Sankai Y, Ohno H, Haga S, Takemasa T (2006) Muscle type-specific response of PGC-1 alpha and oxidative enzymes during voluntary wheel running in mouse skeletal muscle. Acta Physiol (Oxf) 188:217–223
Yoshida Y, Hatta H, Kato M, Enoki T, Kato H, Bonen A (2004) Relationship between skeletal muscle MCT1 and accumulated exercise during voluntary wheel running. J Appl Physiol 97:527–534
Benton CR, Yoshida Y, Lally J, Han XX, Hatta H, Bonen A (2008) PGC-1alpha increases skeletal muscle lactate uptake by increasing the expression of MCT1 but not MCT2 or MCT4. Physiol Genomics 35:45–54
Paulsen SR, Rubink DS, Winder WW (2001) AMP-activated protein kinase activation prevents denervation-induced decline in gastrocnemius GLUT-4. J Appl Physiol 91:2102–2108
Brault JJ, Jespersen JG, Goldberg AL (2010) Peroxisome proliferator-activated receptor gamma coactivator 1alpha or 1beta overexpression inhibits muscle protein degradation, induction of ubiquitin ligases, and disuse atrophy. J Biol Chem 285:19460–19471
Jorgensen SB, Treebak JT, Viollet B, Schjerling P, Vaulont S, Wojtaszewski JF, Richter EA (2007) Role of AMPK alpha 2 in basal, training-, and AICAR-induced GLUT4, hexokinase II, and mitochondrial protein expression in mouse muscle. Am J Physiol Endocrinol Metab 292:E331–E339
Bartlett JD, Hwa Joo C, Jeong TS, Louhelainen J, Cochran AJ, Gibala MJ, Gregson W, Close GL, Drust B, Morton JP (2012) Matched work high-intensity interval and continuous running induce similar increases in PGC-1alpha mRNA, AMPK, p38, and p53 phosphorylation in human skeletal muscle. J Appl Physiol 112:1135–1143
Clark SA, Chen ZP, Murphy KT, Aughey RJ, McKenna MJ, Kemp BE, Hawley JA (2004) Intensified exercise training does not alter AMPK signaling in human skeletal muscle. Am J Physiol Endocrinol Metab 286:E737–E743
Jager S, Handschin C, St-Pierre J, Spiegelman BM (2007) AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA 104:12017–12022
Nagatomo F, Fujino H, Kondo H, Kouzaki M, Gu N, Takeda I, Tsuda K, Ishihara A (2012) The effects of running exercise on oxidative capacity and PGC-1alpha mRNA levels in the soleus muscle of rats with metabolic syndrome. J Physiol Sci 62:105–114
Leick L, Fentz J, Bienso RS, Knudsen JG, Jeppesen J, Kiens B, Wojtaszewski JF, Pilegaard H (2010) PGC-1{alpha} is required for AICAR-induced expression of GLUT4 and mitochondrial proteins in mouse skeletal muscle. Am J Physiol Endocrinol Metab 299:E456–E465
Semenza GL, Roth PH, Fang HM, Wang GL (1994) Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem 269:23757–23763
Ullah MS, Davies AJ, Halestrap AP (2006) The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem 281:9030–9037
Lee M, Hwang JT, Lee HJ, Jung SN, Kang I, Chi SG, Kim SS, Ha J (2003) AMP-activated protein kinase activity is critical for hypoxia-inducible factor-1 transcriptional activity and its target gene expression under hypoxic conditions in DU145 cells. J Biol Chem 278:39653–39661
Ouchi N, Shibata R, Walsh K (2005) AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ Res 96:838–846
Hashimoto T, Hussien R, Oommen S, Gohil K, Brooks GA (2007) Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J 21:2602–2612
Baker SK, Tarnopolsky MA, Bonen A (2001) Expression of MCT1 and MCT4 in a patient with mitochondrial myopathy. Muscle Nerve 24:394–398
Young ME, Radda GK, Leighton B (1996) Activation of glycogen phosphorylase and glycogenolysis in rat skeletal muscle by AICAR–an activator of AMP-activated protein kinase. FEBS Lett 382:43–47
Aschenbach WG, Hirshman MF, Fujii N, Sakamoto K, Howlett KF, Goodyear LJ (2002) Effect of AICAR treatment on glycogen metabolism in skeletal muscle. Diabetes 51:567–573
Holmes BF, Kurth-Kraczek EJ, Winder WW (1999) Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J Appl Physiol 87:1990–1995
Pilegaard H, Juel C (1995) Lactate transport studied in sarcolemmal giant vesicles from rat skeletal muscles: effect of denervation. Am J Physiol 269:E679–E682
McCullagh KJ, Poole RC, Halestrap AP, O’Brien M, Bonen A (1996) Role of the lactate transporter (MCT1) in skeletal muscles. Am J Physiol 271:E143–E150
Kitaoka Y, Masuda H, Mukai K, Hiraga A, Takemasa T, Hatta H (2011) Effect of training and detraining on monocarboxylate transporter (MCT) 1 and MCT4 in Thoroughbred horses. Exp Physiol 96:348–355
Benton C, Campbell S, Tonouchi M, Hatta H, Bonen A (2004) Monocarboxylate transporters in subsarcolemmal and intermyofibrillar mitochondria. Biochem Biophys Res Commun 323:249–253
Acknowledgments
Y. Kitaoka and Y. Takahashi are the recipients of grant-in-aid for JSPS Fellows from the Japan Society for the Promotion of Science.
Conflict of interest
There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kitaoka, Y., Takahashi, Y., Machida, M. et al. Effect of AMPK activation on monocarboxylate transporter (MCT)1 and MCT4 in denervated muscle. J Physiol Sci 64, 59–64 (2014). https://doi.org/10.1007/s12576-013-0290-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12576-013-0290-7