Abstract
Indirect lymphatic system imaging is essential for diagnosing lymphatic diseases. The basic methodology involves intradermal or subcutaneous injection of a contrast agent into the surrounding lymphatic capillary, and the flow of the contrast agent is identified using a detector. Many contrast agents that use near-infrared dye, including indocyanine green (ICG) fluorescent lymphography, are available. ICG is rapidly spreading as a convenient and safe lymphedema diagnostic method, because it does not involve radiation exposure, and the imaging equipment is more compact than other devices. The lymphatic system is a semi-open circulatory system with numerous lymphatic capillaries acting as blind ends. Anatomical information on the injection site and observation of specific lymphatic vessels and nodes is important. However, this anatomical information is lacking. Recent reports suggest that ICG fluorescent lymphography can be applied to cadavers in the same manner as living bodies. Furthermore, these reports have demonstrated the functional aspects of the capillary lymph vessel networks as well as their relationship with lymphatic vessels and lymph nodes. This review article describes the historical progression from the old to the new functional lymphatic anatomy and introduces a new functional lymphography technique for the lower limbs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction: past lymphatic anatomy and lymphatic imaging
Many anatomists have studied the gross anatomy of the lower limb lymphatic system since its discovery in the 1600s. The detailed and artistic drawings of Italian anatomist Paolo Mascagni became the basis of the lymphatic anatomy landmarks (Mascagni. 1787). Around the 1800–1900s, French anatomist Marie Philibert Constant Sappey and his students compiled nearly 100 years of work, collecting anatomical and systematic data. This work included the classification and anatomical details of the collective lymphatic vessels, including their course and position (Sapppey 1874). This information currently remains the basis for lymphatic anatomy. In the 1900s, Japanese anatomists Buntrou Adachi and Takusaburou Kihara described the detailed anatomy of minor regions, including the deep lymphatics (Adachi et al. 1953; Kutsuna 1968). In recent years, there has been growing interest in studying the lymphatic territory of the skin due to the widespread use of lymphatic drainage techniques for lymphedema. Stefan Kubik was the first to describe the relationship between each collecting lymphatic vessel and the lymphatic territories of the skin (Kubik 1969, 1972, 1973, 1980, 1975; Kubik and Manestar 1995). Until the 2000s, the summary of macroscopic lymphatic anatomy had been completed. However, detailed information was lacking regarding the classification of lymphatic vessels and nodes, the appearance rate of each vessel as well as node, and the anomaly rates. Furthermore, the functional relation between lymphatic capillaries and vessels with the lymph nodes (LNs) in the lower extremities was unknown as lymphatic vessel dissection was a time-consuming process, and conducting the study with multiple cadavers was difficult (Suami et al. 2005; Schacht et al. 2009; Yamazaki et al. 2013).
Lymphatic system imaging has only recently become possible in patients. Lymphatic system visualization can be done via direct methods (Jacobson and Johhanson 1959), wherein a contrast agent is injected directly into the collecting lymph vessels using needle cannulation. Additionally, indirect methods (Witte et al. 2000) involve injecting contrast near the lymphatic capillary lymph vessels in the subcutaneous tissue, which is indirectly taken up by the collecting lymph vessels. In the 1950s, with nuclear medicine advancements, lymphoscintigraphy expanded using albumin or colloids bound to radioisotopes. These were injected subcutaneously and indirectly taken up by the collecting lymph vessels. Despite the low detector resolution of gamma rays and the radiation exposure, lymphoscintigraphy remains the gold standard for lymphatic imaging worldwide. Fluorescent lymphography using the near-infrared dye indocyanine green (ICG) was recently developed in Japan and is spreading worldwide due to its high resolution and limited radiation exposure (Kitai et al 2005; Unno et al. 2008; Akita et al 2020, 2022).
Functional lymphatic anatomy
The lymphatic system is a semi-open system of vessels, and lymphography can only visualize the lymphatic system that has taken up the contrast agents. Therefore, it is necessary to identify the independent lymphatic units composed of skin lymphatic capillary territory, collecting vessels, and LNs to be evaluated in lymphography. However, several lymph nodes are in the lower extremities, including the groin and below the knee. Since each lymph node is connected to numerous lymphatic vessels, the number and details of independent lymphatic skin territories in the lower limb are unknown.
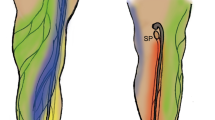
Thus, ICG fluorescent lymphography was initially applied to lower extremity cadaver studies to shorten dissection time, elucidate the functional network aspects of lymphatic capillaries, and establish their relationship with the lymphatic vessels (Shinaoka et al. 2018a, b). The study demonstrated that the lymphatic system from the lower extremity periphery has four independent pathways with different anatomical features: anterolateral (AL), anteromedial (AM), posterolateral (PL), and posteromedial (PM) (Shinaoka et al. 2019) (Fig. 1). Of the four pathways, PL and PM were the main pathways that traveled to the main trunk of the small and great saphenous veins, respectively. However, the AM and AL pathways were minor, traveling to the branching veins of the great saphenous vein (Fig. 2). Additionally, the skin areas were identified where the four lymphatic systems were distributed. In the second study, Computed tomography (CT) lymphangiography demonstrated the close relation of the four lymphatic vessel groups with two lymph nodes in the inguinal region and one in the popliteal (Shinaoka et al. 2020).
Injection site, lymphatic vessel groups, and lymph nodes (reproduced from Shinaoka et al. 2022). Collecting lymphatic vessel groups (posteromedial [PM]: yellow, posterolateral [PL]: red, anteromedial [AM]: blue, and anterolateral [AL]: green) are demonstrated; the PM runs along the main trunk of the great saphenous vein; the PL runs along the main trunk of the small saphenous vein; and the AL as well as the AM run along the branch of the great saphenous vein. Five injection sites (lateral malleolus, medial malleolus, first interdigit space, 4th interdigit space, and lateral foot) and each lymphatic vessel group were visualized, as demonstrated in the radar chart (below). When ICG was injected from each lymphatic group, it reached the lymph nodes as revealed in the upper radar chart (upper): IM inframedial, IL infralateral, SM supramedial, SL supralateral, SP superficial popliteal
Relationship between the lymphatic vessel groups and veins according to the transverse section of the lower leg. The PL is associated with the main trunk of the small saphenous vein, the PM with the main trunk of the great saphenous vein, and the AM with a branch of the great saphenous vein, SSV small saphenous vein, GSV great saphenous vein. Posteromedial [PM]: yellow, posterolateral (PL): red, anteromedial [AM]: blue, and anterolateral [AL]: green
In conclusion, these two cadaveric lower extremity studies revealed the functional aspects of each unit composed of lymphatic capillaries, collecting vessels, and regional LNs. Furthermore, these results led to the establishment of a recommended injection site for evaluating collecting vessels and LNs of the lower extremity. The combination of four injection points (Fig. 3: sites 1 for PM, 7 for AM, 14 for AL, and 16 for PL) can be considered tracer injection sites for mapping all four lymphatic vessel groups. Additionally, three injection points (Fig. 3: sites 7, 14, and 16) were used for mapping regional LNs in the lower extremity.
Recommended injection sites. Nineteen injection sites have been identified. All of these injection sites are located at the border between the dorsal and plantar feet, while the most recommended injection sites are just below the medial malleolus for posteromedial (site 1), just below the lateral malleolus for posterolateral (site 16), the first interdigit space for anteromedial (site 7), and the midpoint of the external popliteal and head fifth metatarsal bone for anterolateral (site 14)
Methodology of ICG lymphography based on lymphatic anatomy
The purpose of lymphography for patients is to determine which lymphatic pathways remain functional as well as which are obstructed, identify lymphatic flow leakage locations into the skin [dermal backflow (DB)], and trace the final lymph flow destination (Shinaoka et al. 2017: Suami et al. 2018; Yamamoto et al. 2011) (Fig. 4). Thus, an accurate diagnosis requires evaluation of the whole lymphatic system in the lower limb.
In the lower extremities, the first interdigital space is the most common tracer injection site (Bourgeois 2007; Notohamiprodjo et al. 2012). However, injection sites using the second interdigital space (Gloviczki et al. 1989) and multiple interdigital spaces have also been reported (Szuba et al. 2003; Lohrmann et al. 2006; Maegawa et al. 2010). According to the latest results (Shinaoka et al. 2019; Shinaoka et al. 2020), toe web space injections only demonstrated the AM pathway while missing the other three pathways. Thus, three additional injection sites are required for analyzing the PM, AL, and PL groups. Lymphangiography textbooks recognize the PL and AL pathways as independent pathways from the AM pathway and recommend cannulating and injecting these three pathways separately (Kimonth. 1952).
If the DB expands immediately after injection, it hides the remaining lymphatic vessels, making ascertainment difficult. The patient was asked to rest after the injection to prevent obscuring the remaining vessels, and only the injection site was massaged with the fingertips. After the ICG has been taken up by the lymphatic vessels, gentle massage along the lymphatic vessels with the fingers was performed to aid the ICG ascent to the lymph nodes, allowing us to evaluate the entire lymphatic flow. However, if the DB only begins to appear, stop massaging the area and focus on stimulating other lymphatic vessels as much as possible to obtain an overall picture. Subsequently, mapping is performed by massaging the lymphatic vessels where the DBs appear.
Exercise stress is usually added to accurately assess the DB appearance and extent (Engeset et al. 1977; Gloviczki et al. 1989; Havas et al. 1997). As DB often appears near the inguinal area, the examination cannot be completed without ICG flow to this area. However, due to the slow flow of lymphatic fluid, 15 min of walking exercise has been reported to be necessary for promoting lymphatic flow and allowing ICG movement to the inguinal region (Matsumoto et al. 2019). The order of ICG injection is also important as the primary PM and PL lymphatic vessels run over the deep fascia, making it difficult to observe them if the AL and AM, which extend into the shallow subcutaneous layers, are contrasted first. Due to this, ICG is first injected to target the PM and PL (Fig. 3: site 1 and 16) to confirm the overall flow and subsequently injected to target the AL and AM (Fig. 3: site 7 and 14). Confirming the presence of PM and PL is crucial as they tend to be deficient in the early lymphedema stages, ensuring an accurate diagnosis.
Interpretation of ICG lymphography based on lymphatic anatomy
It is necessary to confirm the presence and extent of DB (Yamamoto et al. 2011) and the lymphatic pathway defects (Kimonth. 1952) to diagnose lymphedema and assess its severity. The presence or absence of DB is the most important criterion in diagnosing lymphedema. DB commonly appears in the inguinal region but may also appear peripherally, solely at the injection site, or within one lymphatic pathway. Therefore, it is essential to initially contrast the entire area and meticulously observe every detail. If edema other than lymphedema is present at the injection site, ICG may spread subcutaneously, potentially causing a vague diagnosis. In such cases, the diagnosis of DB is confirmed only when there is an observation of ICG spreading into the capillary lymphatics under high power magnification.
While there is a relationship between the appearance of DB and lymphatic pathway defects, these two often occur independently and are separate findings. Since the four lymphatic pathways are almost always present in the normal state, these defects may indicate changes in the lymphatic system. Previous studies have reported that lymphatic pathway defects are strongly associated with lymphedema severity (Shinaoka et al. 2022). In normal-to-mild lymphedema, there is no lymphatic pathway defect. When the PL or PM is defective, the severity increases by one step to mild lymphedema. When the PM and PL are defective, the severity increases by another step. AM and AL often remain intact in severe lymphedema; however, when AM and AL are deficient, lymphedema progresses to the most severe type. Given these results, a severity classification was established to evaluate lymphedema, known as the Lymphatic Pathway defect (LPad) (Fig. 5).

(Reproduced from Shinaoka et al. 2022). Indocyanine green lymphographic images of the four legs with lymphedema depicting four colored lymphatic vessel groups and dermal backflow: anteromedial (blue), anterolateral (green), posteromedial (yellow), and posterolateral (red). Double defects in the posteromedial and posterolateral groups were more severe than the single defects of these two groups. Defects in all lymphatic groups, including the anteromedial and anterolateral groups, were more severe than double defects. Previous reports have revealed that in the terminal stage of leg lymphedema, all lymphatic collecting vessels in the legs are lost
The lymphatic pathway defect (LPad) severity classification in lower extremity lymphedema.
Conclusion
A new functional lymphatic anatomy was added to the previous one, leading to the establishment of a lymphography methodology in the lower extremity from an anatomical perspective. The limitations of the functional lymphatic anatomy include the lack of information about deep lymphatics. Accordingly, additional study about anatomy of deep lymphatics will be needed for further understanding of lymphedema pathology
Data availability
The data that support the findings of this study are available from the corresponding author, [A.S.], upon reasonable request.
References
Adachi B (1953) Das Lymphgefäßsystem der Japaner. Kyoto University, Kyoto
Akita S, Unno N, Maegawa J, Kimata Y, Fukamizu H, Yabuki Y, Shinaoka A, Sano M, Kawasaki Y, Fujiwara T, Hanaoka H, Mitsukawa N (2020) HAMAMATSU-ICG study: Protocol for a phase III, multicentre, single-arm study to assess the usefulness of indocyanine green fluorescent lymphography in assessing secondary lymphoedema. Contemp Clin Trials Commun 19:100595
Akita S, Unno N, Maegawa J, Kimata Y, Fukamizu H, Yabuki Y, Kitayama S, Shinaoka A, Yamada K, Sano M, Ota Y, Ohnishi F, Sakuma H, Nuri T, Ozawa Y, Shiko Y, Kawasaki Y, Hanawa M, Fujii Y, Imanishi E, Fujiwara T, Hanaoka H, Mitsukawa N (2022) A phase III, multicenter, single-arm study to assess the utility of indocyanine green fluorescent lymphography in the treatment of secondary lymphedema. J Vasc Surg Venous Lymphat Disord 10:728–737
Bourgeois P (2007) Scintigraphic investigations of the lymphatic system: the influence of injected volume and quantity of labeled colloidal tracer. J Nucl Med 48:693–695
Engeset A, Olszewski W, Jaeger PM, Sokolowski J, Theodorsen L (1977) Twenty-four hour variation in flow and composition of leg lymph in normal men. Acta Physiol Scand 99:140–148
Gloviczki P, Calcagno D, Schirger A, Pairolero PC, Cherry KJ, Hallett JW, Wahner HW (1989) Noninvasive evaluation of the swollen extremity: experiences with 190 lymphoscintigraphic examinations. J Vasc Surg 9:683–689
Havas E, Parviainen T, Vuorela J, Toivanen J, Nikula T, Vihko V (1997) Lymph flow dynamics in exercising human skeletal muscle as detected by scintography. J Physiol 504:233–239
Jacobson S, Johhanson S (1959) Normal roentgen anatomy of the lymph vessels of upper and lower extremities. Acta Radiol 51:321–328
Kimonth JB (1952) Lymphangiography in man; a method of outlining lymphatic trunks at operation. Clin Sci 11:13–20
Kitai T, Inomoto T, Miwa M, Shikayama T (2005) Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer 12:211–215
Kubik S (1969) Anatomy of the lymphatic system with special reference to lymph node drainage area of the lower part of the body. Strahlentherapie Sonderb 69:8–17
Kubik S (1972) Early development of popliteal lymph nodes. Verh Anat Ges 67:589
Kubik S (1973) Anatomy of the lymphatic system. Radiol Clin Biol 42:243–257
Kubik S (1975) Clinical anatomy of the lymphatic system. Verh Anat Ges 69:109–116
Kubik S (1980) The role of the lateral upper arm bundle and the lymphatic watersheds in the formation of collateral pathways in lymphedema. Acta Biol Acad Sci Hung 31:191–200
Kubik S, Manestar M (1995) Topographic relationship of the ventromedial lymphatic bundle and the superficial inguinal nodes to the subcutaneous veins. Clin Anat 8:25–28
Kutsuna M (1968) Anatomie des lymphsystems der Japaner. Kanehara Shuppan Co, Tokyo, Kyoto, pp 127–137
Lohrmann C, Foeldi E, Speck O, Langer M (2006) High-resolution MR lymphangiography in patients with primary and secondary lymphedema. AJR Am J Roentgenol 187:556–561
Maegawa J, Mikami T, Yamamoto Y, Satake T, Kobayashi S (2010) Types of lymphoscintigraphy and indications for lymphaticovenous anastomosis. Microsurgery 30:437–442
Mascagni P (1787) Vasorum Lymphaticorum Corporis Humani Historia et Ichonographia. Sienne: P. Carli
Matsumoto K, Shinaoka A, Yamada K, Kimata Y (2019) Exercise-loaded indocyanine green fluorescence lymphangiography for diagnosing lymphedema. J Reconstr Microsurg 35:138–144
Notohamiprodjo M, Weiss M, Baumeister RG, Sommer WH, Helck A, Crispin A, Reiser M, Karin H (2012) MR lymphangiography at 3.0 T: Correlation with lymphoscintigraphy. Radiology 264:78–87
Sappey PC (1874) Anatomie, Physiologie, Pathologie des Vaisseaux Lymphatiques. Paris: Adrien Delahaye. https://www.biusante.parisdescartes.fr/histoire/medica/resultats/index.php?do=livre&cote=02094. Accessed 24 Apr 2023.
Schacht V, Luedemann W, Abels C, Berens von Rautenfeld D (2009) Anatomy of the subcutaneous lymph vascular network of the human leg in relation to the great saphenous vein. Anat Rec (hoboken) 292:87–93
Shinaoka A, Koshimune S, Yamada K, Matsumoto K, Honda M, Miyake M, Furuichi H, Hongo A, Kimata Y (2017) Accelerated lymph flow in early-stage secondary lymphedema detected by indocyanine green fluorescence lymphography. J Reconstr Microsurg 33:596–602
Shinaoka A, Koshimune S, Yamada K, Kumagishi K, Suami H, Kimata Y, Ohtsuka A (2018a) A fresh cadaver study on indocyanine green fluorescence lymphography: a new whole-body imaging technique for investigating the superficial lymphatics. Plast Reconstr Surg 141:1161–1164
Shinaoka A, Unno N, Maegawa J, Kimata Y, Akita S, Fujiwara T (2018b) Current usage of imaging modalities for treatment of lymphedema in clinical practice: questionnaire survey of certified institutes of Japan society of plastic and reconstructive surgery. Lymphology (japanese) 41:81–85
Shinaoka A, Koshimune S, Yamada K, Kumagishi K, Suami H, Kimata Y, Ohtsuka A (2019) Correlations between tracer injection sites and lymphatic pathways in the leg: a near-infrared fluorescence lymphography study. Plast Reconstr Surg 4:634–642
Shinaoka A, Koshimune S, Suami H, Yamada K, Kumagishi K, Boyages J, Kimata Y, Ohtsuka A (2020) Lower-limb lymphatic drainage pathways and lymph nodes: a CT lymphangiography cadaver study. Radiology 294:223–229
Shinaoka A, Kamiyama K, Yamada K, Kimata Y (2022) A new severity classification of lower limb secondary lymphedema based on lymphatic pathway defects in an indocyanine green fluorescent lymphography study. Sci Rep 12:309
Suami H, Taylor GI, Pan WR (2005) A new radiographic cadaver injection technique for investigating the lymphatic system. Plast Reconstr Surg 115:2007–2013
Suami H, Koelmeyer L, Mackie L, Boyages J (2018) Patterns of lymphatic drainage after axillary node dissection impact arm lymphoedema severity: a review of animal and clinical imaging studies. Surg Oncol 27:743–750
Szuba A, Shin WS, Strauss HW, Rockson S (2003) The third circulation radionuclide lymphoscintigraphy in the evaluation of lymphedema. J Nucl Med 44:43–57
Unno N, Nishiyama M, Suzuki M, Yamamoto N, Inuzuka K, Sagara D, Tanaka H, Konno H (2008) Quantitative lymph imaging for assessment of lymph function using indocyanine green fluorescence lymphography. Eur J Vasc Endovasc Surg 36:230–236
Witte CL, Witte MH, Unger EC, Williams WH, Bernas MJ, McNeill GC, Stazzone AM (2000) Advances in imaging of lymph flow disorders. Radiographics 20:1697–1719
Yamamoto T, Narushima M, Doi K, Oshima A, Ogata F, Mihara M, Koshima I, Mundinger G (2011) Characteristic indocyanine green lymphography findings in lower extremity lymphedema: the generation of a novel lymphedema severity staging system using dermal backflow patterns. Plast Reconstr Surg 127:1979–1986
Yamazaki S, Suami H, Imanishi N, Aiso S, Yamada M, Jinzaki M, Kuribayashi S, Chang DW, Kishi K (2013) Three-dimensional demonstration of the lymphatic system in the lower extremities with multi-detector-row computed tomography: A study in a cadaver model. Clin Anat 26:258–266
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science through the Grants-in-Aid for Scientific Research (Award Nos. 19H03814, 20H04052, and 22H03249). The author sincerely thanks those who donated their bodies to science, so that anatomical research could be performed. The results from such research can potentially increase our overall knowledge; thus, improving patient care. Therefore, these donors and their families deserve our highest gratitude.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The content of this manuscript was exclusively contributed and written by the authors listed. Akira Shinaoka is a member of the Joint Research Department supported by Hamamatsu Photonics K.K. and TECHNO TAKATSUKI CO., LTD. The authors declare no other conflicts of interest. No ghostwriters were involved in the completion of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shinaoka, A. A new lymphography protocol and interpretation principles based on functional lymphatic anatomy in lower limb lymphedema. Anat Sci Int 99, 153–158 (2024). https://doi.org/10.1007/s12565-023-00754-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-023-00754-2