Abstract
Brain computation relies on the neural networks. Neurons extend the neurites such as dendrites and axons, and the contacts of these neurites that form chemical synapses are the biological basis of signal transmissions in the central nervous system. Individual neuronal outputs can influence the other neurons within the range of the axonal spread, while the activities of single neurons can be affected by the afferents in their somatodendritic fields. The morphological profile, therefore, binds the functional role each neuron can play. In addition, synaptic connectivity among neurons displays preference based on the characteristics of presynaptic and postsynaptic neurons. Here, the author reviews the “spatial” and “temporal” connection selectivity in the neocortex. The histological description of the neocortical circuitry depends primarily on the classification of cell types, and the development of gene engineering techniques allows the cell type-specific visualization of dendrites and axons as well as somata. Using genetic labeling of particular cell populations combined with immunohistochemistry and imaging at a subcellular spatial resolution, we revealed the “spatial selectivity” of cortical wirings in which synapses are non-uniformly distributed on the subcellular somatodendritic domains in a presynaptic cell type-specific manner. In addition, cortical synaptic dynamics in learning exhibit presynaptic cell type-dependent “temporal selectivity”: corticocortical synapses appear only transiently during the learning phase, while learning-induced new thalamocortical synapses persist, indicating that distinct circuits may supervise learning-specific ephemeral synapse and memory-specific immortal synapse formation. The selectivity of spatial configuration and temporal reconfiguration in the neural circuitry may govern diverse functions in the neocortex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the fundamental characteristics in the brain is the synaptic connections among various neurons. Neuronal signal outputs can influence the activity of a finite neuronal population via chemical synapses that are formed in contact sites between them. Neurons spread neurites such as dendrites and axons, and this morphological property increases the surface area and the chance of contacts, resulting in thousands of synaptic inputs to single neurons. Dendrites and axons of neurons do not simply radiate in all directions from their somata; rather, individual neurons display unique ramification patterns (Markram et al. 2004; Xu et al. 2006; Ascoli et al. 2008; Uematsu et al. 2008; Kubota 2014; Feldmeyer et al. 2018; Gouwens et al. 2020). Since this diversity of neuronal morphologies would reflect the functional properties of individual cell populations, the wiring diagrams composed of the synaptic connections should be depicted based on the presynaptic and postsynaptic identities of highly diverse neurons.
Since Cajal (1911) and Lorente de Nó (1938) described qualitative wiring diagrams, the architecture of neural circuits in the neocortex has been quantified through the developments of neuronal labeling techniques such as immunohistochemistry, neuronal tracers, virus vectors and transgenic animals. The morphological characteristics of neurons well correspond to the intrinsic molecular and electrophysiological properties (Markram et al. 2004; Xu et al. 2006; Ascoli et al. 2008; Kubota 2014), indicating that the functional roles neurons can play depend on these cell type profiles. Therefore, the quantitative wiring diagram of the neocortex primarily requires the classification of neuronal subtypes (Fig. 1). In addition, the development of gene engineering techniques allows the cell type-specific visualization of the entire somatodendritic and axonal arborization (Fig. 1). The classification and labeling tools have expedited the anatomical investigation of the wiring principles in the neocortex.
Roadmap for depicting spatiotemporal selectivity of synaptic configuration and reconfiguration. Depicting the diagram of the neocortical network requires cell type classification. Connectivity among cell subtypes at a cellular level can be recorded by electrophysiology. Observation of synaptic configuration at a subcellular resolution needs visualization of somatodendritic and axonal morphology of individual neurons. The subcellular synaptic layout can be rearranged by learning, showing formation, maintenance and elimination of synapses
Cell-to-cell connections can be identified by electrophysiological recordings (Fig. 1). Virus vectors such as herpes simplex virus (Zhang et al. 2015), rabies virus (Kelly and Strick 2000), vesicular stomatitis virus (van den Pol et al. 2009), or adeno-associated virus (AAV) (Zingg et al. 2017) may be applicable for trans-synaptic tracings, though the mechanism and specificity of trans-synaptic transmission remain hypothetical and controversial with potential caveats (Beier 2019). High-resolution microscopy such as electron microscopy verifies the presence of synaptic connections, and anatomical observation at a synaptic spatial resolution provides the information of the subcellular synaptic layout (Fig. 1). For instance, the subcellular distribution of γ-aminobutyric acid-ergic (GABAergic) inputs in glutamatergic pyramidal cells has been well established (Somogyi et al. 1998; Thomson and Bannister 2003; Jiang et al. 2013; Kubota 2014; Kubota et al. 2015, 2016): parvalbumin-expressing (PV+) basket cells with a fast-spiking firing property, one of the major GABAergic neuronal populations, are reported to largely innervate the perisomatic domain of pyramidal cells, while the other GABAergic cell types such as somatostatin-expressing (SOM+) neurons preferentially target the dendrites. Likewise, glutamatergic and GABAergic synaptic convergence in GABAergic neurons also shows compartmentalized subcellular innervation in a presynaptic cell type-dependent manner (Kameda et al. 2012; Hioki et al. 2013, 2018; Sohn et al. 2016). This spatial selectivity of subcellular innervation patterns indicates presynaptic cell type-specific regulation of single neuron excitability.
Furthermore, synaptic connections are not static but dynamic, and synaptic plasticity is believed to be the cellular basis of learning. In the primary motor cortex (M1), motor learning triggers synaptogenesis in the apical tuft of layer (L) 5 pyramidal cells (Xu et al. 2009; Yang et al. 2009; Wang et al. 2011; Fu et al. 2012). The apical tufts of pyramidal cell dendrites are located in L1, and excitatory afferents in L1 of the neocortex consist of both cortical and thalamic axon fibers (Kuramoto et al. 2009, 2015; Kaneko 2013; Ueta et al. 2014; Shigematsu et al. 2016; Hasegawa et al. 2020). We showed that the neighboring corticocortical and thalamocortical synapses display unique dynamics in motor skill learning (Sohn et al. 2022). Identification of presynaptic cell types combined with observation of synaptic dynamics in vivo demonstrates that the synaptic dynamics during learning depend on the origin of presynaptic axons innervating newly generated synapses (Fig. 1). This temporally selective reconfiguration of cortical synapses indicates that corticocortical and thalamocortical synapses play distinct functional roles in learning and memory.
In this review, the author summarizes the spatiotemporal selectivity of the neocortical synaptic wirings. We have anatomically investigated the wiring diagram, “connectome”, composed of diverse cell types in the neocortex, and we have revealed the spatial selectivity of the subcellular synaptic configuration in single cells with fixed brain samples. This neural circuit map of the neocortex, however, lacks the information about the role that each wiring plays in a particular function of animals. We further tackled the issue of how the presynaptic identity might determine the rewiring process in skill learning. Observation of circuit-specific synaptic dynamics may allow us to unveil “functional connectome” involved in animal’s behavior in vivo.
Cell type classification in the neocortex
The neocortex contains a variety of cell types, and the connection selectivity in the neural circuit notably depends on the cell characteristics of presynaptic and postsynaptic neurons. Neocortical neurons can be largely classified into glutamatergic excitatory neurons (~ 80%) and GABAergic inhibitory neurons (~ 20%). Although glutamatergic pyramidal cells abound in morphological diversity (van Aerde and Feldmeyer 2015), they are primarily classified by the laminar location of their somata. The laminar location roughly corresponds to their typical projection targets (Molyneaux et al. 2007; Petreanu et al. 2007; Mao et al. 2011; Han et al. 2018): for example, L2/3 pyramidal cell axons arborize within the telencephalic regions [intratelencephalic (IT) cells], while L5 of the frontal cortex consists of two major subtypes, contralaterally projecting IT cells and pontine nuclei-projecting pyramidal tract (PT) cells (Hallman et al. 1988; Cowan and Wilson 1994; Reiner et al. 2003; Arlotta et al. 2005; Morishima and Kawaguchi 2006; Morishima et al. 2011; Ueta et al. 2014; Kawaguchi 2017). L5 IT cells in the secondary motor cortex (M2) broadly send feedback signals to multiple cortical areas, and contain diverse projection types with different L1-targeting preference (Im et al. 2023). PT cells project to the ipsilateral pontine nuclei (Kita and Kita 2012), and they also innervate the other brain regions outside the telencephalon such as the thalamus, superior colliculus, zona incerta, the other brainstem areas and the spinal cord (Hirai et al. 2012; Kita and Kita 2012). PT cells are responsible for motor commands and preparatory activities, and could further be divided into subclasses by the difference of the major target subcortical regions such as the thalamus and the brainstem (Economo et al. 2018). L5 PT cells in the motor cortex are responsible for oscillatory activity and motor learning compared with IT cells (Otsuka and Kawaguchi 2021).
L2/3 pyramidal cells are also composed of subtypes according to their projection patterns (Little and Carter 2012; Lu et al. 2017; Liu and Carter 2018). For example, we identified two types of L2 pyramidal cells in M2 with different electrophysiological and morphological characteristics: those projecting to the contralateral M2 (cM2) and those projecting to the ipsilateral perirhinal cortex (iPRC) (Ueta et al. 2019). In addition, in the primary somatosensory barrel cortex (S1BF), L2/3 cells can be classified into neurons projecting to M1 and those projecting to the secondary somatosensory area (S2), exhibiting different membrane potential dynamics associated with tactile perception (Yamashita et al. 2013). Therefore, these target-dependent subdivisions may reflect functional diversity among L2/3 cells.
GABAergic cells are also morphologically and electrophysiologically heterogeneous. The morphological and electrophysiological identities well correspond to the gene expression such as calcium-binding proteins and neuropeptides, and their molecular characteristics are utilized for identification of the subpopulations (Kubota et al. 1994, 2011; Kawaguchi and Kubota 1996; Gonchar and Burkhalter 1997; Xu et al. 2006, 2010; Uematsu et al. 2008; Rudy et al. 2011; Hioki et al. 2013; Pfeffer et al. 2013; Kepecs and Fishell 2014; Gouwens et al. 2020). In the mouse neocortex, PV+ and SOM+ neurons account for approximately ~ 40% and ~ 30% of GABAergic neurons, respectively, and remaining ~ 30% of them express ionotropic serotonin receptor 5HT-3a (5HT-3aR) (Rudy et al. 2011; Tremblay et al. 2016; Feldmeyer et al. 2018). PV+ neurons include fast-spiking basket cells and chandelier cells, which innervate the perisomatic region of pyramidal cells, whereas the SOM+ population contains dendrite-targeting Martinotti cells. The 5HT-3aR+ subpopulation includes vasoactive intestinal polypeptide-expressing (VIP+) neurons with vertically oriented dendrites and axons, and non-VIP cells such as neurogliaform cells and L1 interneurons (Lee et al. 2010; Schuman et al. 2019). The significance of the classification based on these molecular profiles has been supported by the fact that individual GABAergic subclasses such as PV+, SOM+ and VIP+ neurons are involved in distinct functions in vivo (Gentet et al. 2012; Lee et al. 2012, 2019; Lovett-Barron et al. 2012; Wilson et al. 2012; Pi et al. 2013; Fu et al. 2014; Kepecs and Fishell 2014; Zhang et al. 2014; Kamigaki and Dan 2017; Abbas et al. 2018; Adler et al. 2019; Xu et al. 2019; Yu et al. 2019).
The GABAergic cell type taxonomy as above was reproducible by recent large-scale transcriptomic studies (Zeisel et al. 2015; Tasic et al. 2016; Gouwens et al. 2020; Sun et al. 2021) and also applicable to the human neocortex (Hodge et al. 2019). These datasets with systematic high-throughput measurements and analyses, however, have substantiated the further diversity in the previously defined cell subclasses. In fact, SOM+ axonal ramification patterns are diverse in S1BF (Tremblay et al. 2016; Nigro et al. 2018) and morphologically distinct SOM+ subtypes exhibit opposite activity changes that are dependent on the laminar locations and the brain state (Muñoz et al. 2017). In addition, SOM+ neurons are diverse in gene expression, which can be subdivided into calbindin-, calretinin-, neuropeptide Y-, neuronal nitric oxide synthase-, and/or nicotinic acetylcholine receptor α2 subunit-expressing neurons (Kubota et al. 1994; Kawaguchi and Kubota 1997; Ma et al. 2006; Xu et al. 2010; Tasic et al. 2016; Hilscher et al. 2017). We showed that preprodynorphin (PPD), the precursor of an opioid peptide dynorphin, was also expressed selectively in SOM+ neurons (Sohn et al. 2014) (Fig. 2), which is consistent with a transcriptomic study (Smith et al. 2019). PPD+ cells are distributed throughout the neocortex (Fig. 2a, b), mostly in L4 and L5 (Fig. 2c). Individual PPD+ neurons show multipolar or bipolar somatodendritic morphology (Fig. 2d). PPD-immunoreactive cell bodies co-express SOM (Fig. 2e), indicating SOM+ neurons can be divided into two subtypes by PPD expression (Fig. 2f). Intriguingly, a following study showed that the proportion of PPD+ cells in SOM+ neurons of S1BF can fluctuate dynamically during tactile information-associated learning (Loh et al. 2017), implying that the dynorphin expression level in the neocortex may represent a functional state of SOM+ neurons that are sensitive to developmental and environmental factors underlying human psychiatric disorders (Casello et al. 2022). The morphological, electrophysiological and functional properties of these diverse SOM+ cell subtypes will be further determined via future technical development (Hostetler et al. 2023).
GABAergic neuronal subclasses and characterization of PPD+ cells in the neocortex. a Immunohistochemistry for PPD. b Distribution of PPD+ neurons in the neocortex. PPD+ cell bodies are plotted in a coronal brain section including S1BF. CPu caudate-putamen, GP globus pallidus, HL hindlimb area, ic internal capsule, M1 primary motor area, M2 secondary motor area, Rt thalamic reticular nucleus, S1 primary somatosensory area, S2 secondary somatosensory area, Th thalamic nuclei. c Magnified image of the vertical strip in panel a. wm, white mater. d Multipolar and bipolar somatodendritic morphology of PPD+ neurons. e Double immunofluorescence labeling for PPD (green) and SOM (magenta) in S1BF. Arrowheads indicate PPD+ cell bodies. f Drawing representing the proportion of GABAergic cell subtypes in the neocortex. CR calretinin, NPY neuropeptide Y, NOS neuronal nitric oxide synthase. Modified with permission from Sohn et al. (2014)
Gene engineering for visualization of neuronal morphology
Quantitative anatomical diagrams of synaptic wirings in the neocortex involve complete somatodendritic visualization of postsynaptic cells of interest as well as the information of the presynaptic cell identity. Gene engineering techniques are applicable for Golgi-like staining of particular neuronal subsets. Transgenic or bacterial artificial chromosome (BAC) transgenic mouse lines have been developed for brain-wide expression of fluorescent proteins in a cell type-specific manner (Feng et al. 2000; Ma et al. 2006; Kameda et al. 2012; Kaneko et al. 2018). In utero electroporation allows cell birthday-specific gene transduction in the brain region of interest (Saito and Nakatsuji 2001; Tabata and Nakajima 2001), which is applied for layer-selective cell labeling in the neocortex. Virus vectors, such as lentivirus (Naldini et al. 1996; Zufferey et al. 1997; Miyoshi et al. 1998; Amendola et al. 2005; Cronin et al. 2005; Hioki et al. 2007, 2009; Hirano et al. 2013; Kato and Kobayashi 2020), adenovirus (Niwa et al. 1991; Moriyoshi et al. 1996; Tamamaki et al. 2000; Tomioka and Rockland 2006), canine adenovirus-2 (Soudais et al. 2001; Junyent and Kremer 2015), rabies virus (Kelly and Strick 2000), Sindbis virus (Altman-Hamamdzic et al. 1997; Gwag et al. 1998; Furuta et al. 2001), and AAV (Kaplitt et al. 1994; Tervo et al. 2016; Challis et al. 2022), are available for anterograde and/or retrograde neuronal labeling by local transfection in the vicinity of injection sites (Davidson and Breakefield 2003; Callaway 2005). In addition, transgenic and knock-in mouse lines with expression of recombinases such as Cre and Flp (Taniguchi et al. 2011; Gerfen et al. 2013; Daigle et al. 2018) are employed for cell type-specific labeling in combination with reporter mice or virus vectors (Livet et al. 2007; Madisen et al. 2010; He et al. 2016; Daigle et al. 2018). The availability of these genetic tools has been accelerating the exploration of the neural network architecture.
Recombinant AAV vectors are now widely applied for neuroscience due to their high gene delivery efficiency and low toxicity. Although the human synapsin I (hSyn) promoter-driven gene expression guarantees neuron-selective labeling of both glutamatergic and GABAergic cells, its expression level is insufficient compared with the control of ubiquitous promotors such as the human cytomegalovirus (CMV) and CMV early enhancer/chicken β actin (CAG) promoters (Lukashchuk et al. 2016). To achieve both neuron selectivity and efficiency of gene transduction, we developed a hybrid type AAV platform, AAV-SynTetOff (Sohn et al. 2017); an improved version of tetracycline-controlled transactivator (tTAad) is driven by the hSyn promoter for neuron-selective expression, while a reporter gene is strongly expressed under the tetracycline-responsive element (TRE) promoter to which tTAad binds (Fig. 3a). This AAV-SynTetOff system induces high-level transgene expression selectively in neurons. The expression level in cultured Neuro-2a cells in vitro was approximately 2- and 15-fold higher with the AAV-SynTetOff system than with the conventional AAV vectors driven by the CMV and hSyn promotors, respectively (Fig. 3b, c). Moreover, this AAV vector implements high-level gene transduction in vivo as well (Fig. 3d): the native fluorescence intensity of green fluorescent protein (GFP) was measured in neostriatal neurons infected by the AAV-SynTetOff-GFP vector, showing that this platform allows 34–43 times higher transduction efficiency than the conventional AAV vectors (Fig. 3e). As expected, the transgene was expressed selectively in neurons (Fig. 3f). The promising high-level transgene expression by AAV-SynTetOff-derived vectors can be applied for deep brain imaging with fluorescent proteins (Furuta et al. 2022), bioluminescence (Iwano et al. 2018) or biosensors (Jones-Tabah et al. 2020). In addition, combined with Cre-driver mouse lines, injection of an AAV-SynTetOff vector equipped with lox sequences recognized by Cre recombinase can strongly label particular cell populations (Sohn et al. 2016, 2017; Furuta et al. 2022). These technical advances can promote the exploration of neuronal morphology and neural network architecture.

Modified from Sohn et al. (2017)
Neuron-specific high-level transgene expression with the AAV-SynTetOff vector. a Construction of the vector plasmids, pAAV-CMV-GFP-BGHpA, pAAV-hSyn-GFP-BGHpA, and pAAV-SynTetOff-GFP. b GFP native fluorescence (NF) by infection of the AAV-SynTetOff-GFP and control vectors in Neuro-2A cells in vitro. c Gene-transduction efficiency of the AAV-SynTetOff-GFP and control vectors. The GFP-NF intensity of cells infected with AAV-CMV-GFP-BGHpA is normalized to 1 arbitrary unit (a.u.). d GFP expression (green) in neostriatal neurons by injection of the AAV-SynTetOff-GFP and two control vectors with immunolabeling for neuron-specific nuclear protein (NeuN, magenta). Arrowheads indicate GFP-expressing neurons, while an arrow indicates a NeuN-negative putative glial cell. e GFP-NF intensities in neostriatal neurons. The mean GFP-NF intensity with AAV-CMV-GFP-BGHpA is normalized to 1. AAV-SynTetOff-GFP transduces much stronger GFP expression in neurons than AAV-CMV-GFP-BGHpA and AAV-hSyn-GFP-BGHpA (factors of 43.3 and 34.3, respectively). f Specificities of GFP expression in neostriatal neurons. AAV2/1-hSyn-GFP-BGHpA and AAV-SynTetOff-GFP display specific expression in neurons, while the expression of GFP with AAV-CMV-GFP-BGHpA is not neuron-specific. Error bars, ± standard error of the mean (s.e.m.). ***P < 0.001, Tukey’s multiple-comparison test after one-way analysis of variance (ANOVA).
Spatially selective subcellular configuration of cortical synapses
A single-cell axonal ramification of a presynaptic neuron restrains the encounter with postsynaptic neurons. Corticocortical (Veinante and Deschênes 2003; Rockland 2020; Peng et al. 2021) and thalamocortical (Arnold et al. 2001; Furuta et al. 2009, 2011; Kuramoto et al. 2009, 2015, 2017; Ohno et al. 2012; Nakamura et al. 2015) excitatory efferents as well as GABAergic interneuron axons (Markram et al. 2004; Ascoli et al. 2008; Uematsu et al. 2008; Kubota 2014; Feldmeyer et al. 2018; Gouwens et al. 2020) have preferential laminar (tangential) and columnar (vertical) targets in the neocortex. In addition, postsynaptic neuron dendrites can uniquely spread: for example, each pyramidal cell has a thick, vertically oriented apical dendrite as well as several basal dendrites around its soma. Using an AAV-SynTetOff vector injected into VIP-Cre mice, we showed that L2/3 VIP+ neurons in S1BF resemble pyramidal cells in somatodendritic morphology (Sohn et al. 2016, 2017). The dendritic morphologies of pyramidal cells and L2/3 VIP+ neurons in S1BF indicate that they are strongly affected by afferents in L1, where top-down feedback signals from the frontal cortices abundantly terminate (Veinante and Deschênes 2003; Lee et al. 2013; Manita et al. 2015). Thus, we can suppose the synaptic connectivity to some extent from the information of the axonal ramification and somatodendritic morphology.
Moreover, presynaptic neurons can show subcellular target preference in postsynaptic neurons. In particular, individual cell types of GABAergic neurons in the neocortex display unique innervation patterns on the subcellular somatodendritic domains of pyramidal cells, such as somata, dendritic shafts, dendritic spines, and axon initial segments (Somogyi et al. 1998; Thomson and Bannister 2003; Jiang et al. 2013; Kubota 2014; Kubota et al. 2015, 2016). PV+ and SOM+ neurons preferentially target the perisomatic and dendritic parts in pyramidal cells, respectively, uniquely affecting the pyramidal cell activity (Gentet et al. 2012; Lee et al. 2012; Lovett-Barron et al. 2012; Kamigaki and Dan 2017; Abbas et al. 2018; Xu et al. 2019). For instance, in the visual cortex, PV+ and SOM+ neurons show divisive and subtractive effects on pyramidal cell responses to visual stimuli (Wilson et al. 2012): the relatively uniform suppression by SOM+ cells leads to sharpening the direction selectivity, whereas the non-uniform inhibition by PV+ neurons results in proportional gain reduction. Although both inhibit the pyramidal cell excitability, the subcellular localization of GABAergic synapses may cause presynaptic cell type-specific modulation of the postsynaptic cell activity.
In addition, GABAergic neurons form reciprocal synaptic connections with each other, and the inhibitory network displays selective cell-to-cell connectivity based on presynaptic and postsynaptic cell types (Pfeffer et al. 2013; Jiang et al. 2015; Karnani et al. 2016). For example, VIP+ cells strongly innervate SOM+ and PV+ neurons (Pfeffer et al. 2013; Pi et al. 2013; Kepecs and Fishell 2014), potentiating the excitability of pyramidal cells via inhibition of other GABAergic cell types. This disinhibition of pyramidal cell activity by VIP+ cells is observable throughout the neocortex, such as the somatosensory (Lee et al. 2013; Karnani et al. 2016), auditory (Pi et al. 2013), visual (Fu et al. 2014; Zhang et al. 2014) and medial prefrontal cortices (Pi et al. 2013; Lee et al. 2019). VIP+ neurons in the sensory cortices are recruited by feedback afferents derived from the motor-associated frontal cortices (Lee et al. 2013; Zhang et al. 2014), and they are in fact activated during animal’s movement (Fu et al. 2014; Yu et al. 2019). This functional property of VIP+ neurons indicates that top-down motor information may modulate the sensitivity of pyramidal cell responses to sensory stimuli via VIP+ neuron-mediated disinhibition. Therefore, the regulation of VIP+ cell activity in the sensory cortices is of particular importance for sensory perception.
Electrophysiological recordings have confirmed both excitatory and inhibitory innervation of VIP+ neurons (Porter et al. 1998; Rozov et al. 2001; Lee et al. 2013; Pfeffer et al. 2013), and perisomatic synapses on VIP+ cells have been morphologically observed with immunohistochemical labeling for VIP (Staiger et al. 1996, 1997, 2002). In the sensory cortices, electron microscopy demonstrated that synapses on VIP+ neuron somata were mostly symmetric (Hajós and Zilles 1988), suggestive of GABAergic inputs: indeed, PV+ axons form abundant synapses on the somatic region of VIP+ cells (Staiger et al. 1997, 2002). However, the electrophysiologically reported postsynaptic events in VIP+ neurons evoked by feedback excitatory and SOM+ inhibitory afferents (Lee et al. 2013; Pfeffer et al. 2013) had not been fully supported by morphological evidence, probably due to insufficient visualization of vertically elongated VIP+ neuron dendrites with immunolabeling for VIP (Fig. 4a). We then applied genetic labeling using an AAV-SynTetOff vector combined with the VIP-Cre mouse line instead of immunostaining (Fig. 4a), and systematically quantified the presynaptic cell type-specific innervation patterns (Sohn et al. 2016) (Fig. 4b). Complete reconstruction of L2/3 VIP+ neuron dendrites in S1BF displayed thick-tufted dendrites in L1. Excitatory synapses on VIP+ neurons were preferentially formed in the distal dendrites of L2/3 VIP+ neurons (Fig. 4b,c), and, in particular, corticocortical glutamatergic axons strongly targeted the distal-dendritic compartment in L1. This morphological investigation of synaptic contacts in VIP+ neurons including their distal dendrites could explain the strong effect of top-down corticocortical afferents on VIP+ neuron activity (Lee et al. 2013; Zhang et al. 2014).

Modified from Sohn et al. (2016)
Spatially selective subcellular configuration of synapses on VIP+ neurons. a Somatodendritic visualization of VIP+ neurons with the injection of an AAV-SynTetOff vector into a VIP-Cre mouse. The AAV vector contains double-floxed inverted open reading frame (DIO) for Cre-dependent expression of GFP. Somatodendritic morphology is clearly visualized compared with VIP-immunoreactivity (ir). Arrowheads indicate GFP-expressing VIP+ neurons. b Densities per surface area (µm2) on VIP+ neurons of excitatory corticocortical (CC) synapses (left) and inhibitory synapses derived from PV+ neurons (right). CB, cell body. c Densities (mean ± s.e.m.) of excitatory CC, thalamocortical (TC) and inhibitory synapses along the somatodendritic axis of VIP+ neurons (left). Inhibitory synaptic densities originating from PV+, SOM+ and VIP+ neurons are separately quantified (right), showing perisomatic (< 100 µm from CB) and distal-dendritic (≥ 100 µm) targeting of PV+ and SOM+ neurons. d Schematic diagram summarizing subcellular distribution of synaptic inputs to VIP+ neurons. Pyr, pyramidal cell.
GABAergic [vesicular GABA transporter-positive (VGAT+)] inhibitory innervation showed seemingly uniform distribution in the somatodendritic axis of VIP+ neurons (Fig. 4c, left). However, GABAergic subclasses displayed unique subcellular layouts of synapses in VIP+ neurons (Fig. 4c, right). PV+ neurons preferentially targeted the perisomatic compartment (within 100 µm from somata) of VIP+ cells (Fig. 4b, c), consistent with previous reports (Staiger et al. 1997, 2002). By contrast, SOM+ inhibitory synapses were observable largely in the distal-dendritic compartment (≥ 100 µm from somata) (Fig. 4c, right), and unidentified GABAergic cells, probably L1 interneurons or neurogliaform cells (Rudy et al. 2011; Jiang et al. 2013; Lee et al. 2015), were assumed to innervate the distal-dendritic compartment in the same manner. This compartmentalized subcellular configuration of GABAergic synapses (Fig. 4d) indicates an inhibitory division of labor in which VIP+ neuron excitability is regulated differently between the perisomatic and distal-dendritic compartments. This presynaptic cell type-dependent preference of subcellular targets corresponds to different postsynaptic properties, such as action potential backpropagation and calcium dynamics, between proximal (< 100 µm) and distal (> 100 µm) dendrites of GABAergic cells due to the presence of IA-type potassium currents (Goldberg et al. 2003). Observation at a subcellular spatial resolution with genetic somatodendritic labeling, therefore, revealed presynaptic cell type-dependent compartments in L2/3 VIP+ neurons.
The cell type-specific subcellular distribution of synapses may be a fundamental principle for activity regulation of single neurons. Indeed, the subcellular innervation pattern of PV+ neurons also depends on presynaptic cell characteristics (Kameda et al. 2012; Hioki et al. 2013, 2018): we generated a BAC transgenic mouse line in which PV+ somatodendritic membrane is selectively labeled with GFP, and described the subcellular layout of synaptic inputs to PV+ neurons in S1BF. The somatic compartment of PV+ neurons was strongly innervated by VIP+ and cholecystokinin+ neurons, while PV+ reciprocal synaptic connections as well as glutamatergic inputs were formed predominantly in their dendritic compartment. These morphological evidences support the function of VIP+ neurons as an upstream element for the disinhibitory circuit in the neocortex (Pfeffer et al. 2013; Pi et al. 2013; Kepecs and Fishell 2014) as well as a presynaptic source directly inhibiting pyramidal cell dendrites (Zhou et al. 2017). In addition to cell type-specific unique laminar and columnar spreads of dendrites and axons, these observations at a subcellular spatial resolution demonstrate non-uniform synaptic configuration in the somatodendritic structures of both pyramidal cells and GABAergic neurons. This “spatial selectivity” of synaptic connections between presynaptic and postsynaptic neurons may cause unique functions of distinct neuronal subpopulations.
Temporally selective reconfiguration of two types of excitatory synapses
The “snapshots” of anatomical diagrams in fixed brain tissues show the spatial selectivity of synaptic wirings. Neural networks, however, can be rearranged, and the synaptic dynamics are thought to underlie animal’s learning. Since excitatory synapses in pyramidal cells are typically formed on dendritic spines, the spine plasticity including formation and elimination reflects synaptic dynamics in the neocortex. Two-photon time-lapse imaging in vivo combined with genetic fluorescence labeling allows long-term observation of the spine turnover in the neocortex (Grutzendler et al. 2002; Trachtenberg et al. 2002), demonstrating the correlation of spine plasticity with learning: for instance, motor learning enhances spinogenesis in pyramidal cell dendrites of M1 (Xu et al. 2009; Yang et al. 2009; Fu et al. 2012; Peters et al. 2014; Chen et al. 2015). Although this enhanced spine turnover in M1 should underlie circuit remodeling in motor skill learning, the circuit-based logic behind the change had remained obscure because the description of postsynaptic spine plasticity lacked the information of the presynaptic neuronal partners innervating those spines (Fig. 5a). The upstream partners of M1 originate from both the cortical and subcortical brain regions such as the higher order motor areas and the motor-related thalamic nuclei (Kuramoto et al. 2009, 2015; Hooks et al. 2013; Kaneko 2013; Ueta et al. 2014; Shigematsu et al. 2016; Kawaguchi 2017; Hasegawa et al. 2020) (Fig. 5a). The temporal principle for the circuit remodeling in learning, therefore, requires identification of the presynaptic origin of inputs to the learning-associated postsynaptic spines.

Modified from Sohn et al. (2022)
Correlated two-photon, confocal and electron microscopy for identification of the presynaptic origin of inputs to new spines formed during learning. a New spine formation in the apical tuft of Thy1-GFP-M mice during a forelimb reaching task. Spinogenesis in M1 coincides with motor skill improvement, but the origin of inputs to the newly formed spines remained to be identified. Both corticocortical and thalamocortical fibers can terminate on the apical tuft. b Two-photon microscopy in vivo followed by post hoc immunohistochemistry for presynaptic (VGluTs) and postsynaptic (Homer1) markers. A newly formed spine is observed in the quadruple fluorescence image. Arrowheads indicate a putative corticocortical (VGluT1+) synaptic input site. Double-arrowheads indicate a thalamocortical (VGluT2+) axon terminal close to the new spine that innervates a different target. Electron microscopy (EM, right) confirms synaptic structure at the putative synaptic input sites indicated by arrowheads.
We solved this problem with a combination of experimental techniques, including in vivo two-photon imaging, post hoc immunohistochemistry, confocal microscopy with image deconvolution, and correlated light and electron microscopy (CLEM) (Sohn et al. 2022) (Fig. 5b). We trained Thy1-GFP-M mice (Feng et al. 2000) to reach for single seeds with their preferred forelimbs, and monitored spine dynamics on the apical tufts of GFP-labeled L5 PT pyramidal cells in the forelimb region of M1 under a two-photon microscope. Spine formation and elimination rates were correlated with the skill improvement, consistent with previous studies (Xu et al. 2009; Yang et al. 2009; Wang et al. 2011; Fu et al. 2012; Hayashi-Takagi et al. 2015). After 4 or 8 days of the training and two-photon imaging, we performed post hoc immunohistochemistry for vesicular glutamate transporters (VGluTs; VGluT1 for cortical axon terminals and VGluT2 for thalamic fibers) as well as a postsynaptic marker Homer1. The confocal microscopy at a synaptic spatial resolution successfully determined the origin of inputs to newly formed spines, and synaptic structures were confirmed on these new spines by CLEM. These combined techniques revealed synaptic and circuit rearrangements that underlie behavioral change in animal’s skill learning (Fig. 6). We showed that corticocortical axon terminals predominantly innervated newly formed spines while only a small population of new spines received thalamocortical inputs during initial 4 days of the task training (Fig. 6a, b). During the subsequent 4 days of the task training, however, new spines receiving the corticocortical input were largely pruned while new spines with the thalamocortical synapses survived and grew in size (Fig. 6a, b). Thus, the correlated two-photon, confocal and electron microscopy revealed presynaptic cell type-dependent “temporal selectivity” of synaptic connections during learning: profuse and transient corticocortical synaptogenesis versus fewer but longer-lasting formation of thalamocortical synapses (Fig. 6b).

Modified from Sohn et al. (2022)
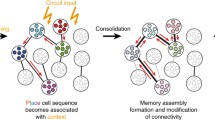
Temporally selective synaptic reconfiguration and circuit remodeling underlying behavior modification. a Behavioral improvement in skill learning. Rapid increase in successful reaches is followed by a slowdown in growth. b Schematic diagram of spine dynamics in M1 during motor learning. New spines formed during initial 4 days of learning are largely innervated by corticocortical (CC) axons. These corticocortically innervated new spines are mostly pruned during the subsequent 4 days, while new spines innervated by thalamocortical (TC) neurons are sustained and enlarged. c Circuit remodeling suggested by synaptic dynamics in M1. Cognitively demanding processes during the early phase of learning involve top-down corticocortical inputs, and the acquired motor skill may be stored long term at thalamocortical innervation sites.
This input type-dependent rewiring process may explain the circuit-based mechanism for transition from attentive to subliminal motor skill improvement (Fig. 6c). New thalamocortically innervated spines were likely to appear in the dendritic segments with new corticocortically innervated spines, and the active spine formation preceded thalamocortical synaptogenesis (Sohn et al. 2022). These spatial and temporal features of new spines in the dendritic segments may explain branch-specific synaptic plasticity during learning (Yang et al. 2014; Cichon and Gan 2015; Xu et al. 2023). L1 of M1 is strongly innervated by the higher order motor area such as M2 (Ueta et al. 2014; Hasegawa et al. 2020), which is responsible for goal-directed behavior and motor planning (Guo et al. 2014; Li et al. 2015; Allen et al. 2017), and its neuronal ensemble activity can be modified by motor learning (Cao et al. 2015; Makino et al. 2017). The motor thalamic nuclei also project to M1, mediating the afferents from the subcortical regions such as the basal ganglia and the cerebellum (Kuramoto et al. 2011), which are associated with automatic and habitual movement. These functions of the presynaptic cells suggest that “ephemeral” corticocortical synapses that appear only during the learning asymptote might provide learning-associated conditioning of the targeted dendritic segments; these teaching contacts may update internal programs for mnemonic transfer to “immortal” thalamocortical synapses generated in the corticocortically supervised segments. This presynaptic cell type-selective reconfiguration may underlie transition from cognitively demanding efforts to subcortically driven automatic motor control.
The unique contribution of corticofugal and thalamofugal pathways to motor learning and execution was also reported in the striatum. Motor learning requires dorsolateral striatum (DLS)-projecting cortical neurons, while DLS-projecting thalamic neurons are essential for executing consolidated motor skills (Wolff et al. 2022). As shown in the striatum, cortical and thalamic afferents in M1 might play distinct roles in motor learning and memory. Indeed, chemogenetic blockade of corticocortical inputs from M2 impaired skill improvement as well as synaptogenesis in M1 (Sohn et al. 2022). On the other hand, daily blockade of thalamocortical inputs seemingly did not directly affect task performance, but thalamic inactivation hindered the enlargement of thalamocortically innervated spines and, instead, enhanced the maturation of corticocortically innervated spines. This compensation by corticocortical synapses may explain the intact performance under thalamocortical silencing. In addition, daily chemogenetic blockade of corticocortical inputs interferes with skill improvement in the early phase of the skill learning, whereas temporary thalamocortical inactivation after motor skill learning disrupted the task performance of the expert mice (Sohn et al. 2022). These inequivalent programs of synaptic dynamics supervised by two distinct afferents suggest that there are two classes of synapses—those specific to learning and those specific to memory. This overturns the widely held assumption that single synapses must necessarily mediate both learning and memory. Since the neocortex is a repeating lattice with presumed common principles, it can be expected that neural circuit-dependent “temporal selectivity” of synaptic reconfiguration we reported in this study (Sohn et al. 2022) may eventually apply to other cortical areas and learning behaviors.
Perspectives
To complete the whole-brain wiring diagram is an extremely challenging project. As mentioned above, the subcellular input patterns have been reported in pyramidal, VIP+ and PV+ cells, and the standard neural circuit has been investigated based on the traditional cell classification. With the advent of large-scale transcriptomics, however, the detailed taxonomy of cortical neurons has been promoted, revealing substantially more diverse cell subtypes than the traditional classification (Zeisel et al. 2015; Tasic et al. 2016; Smith et al. 2019; Gouwens et al. 2020; Sun et al. 2021). The detailed classification will deepen the characterization of the intrinsic morphological and electrophysiological properties as well as the connectivity among these individual subtypes. For instance, SOM+ neurons (Tremblay et al. 2016; Nigro et al. 2018; Smith et al. 2019) and L1 interneurons (Schuman et al. 2019; Meng et al. 2023) can be subdivided particularly by their axonal morphologies, and the convergent inputs to these GABAergic neurons have not been fully investigated anatomically. In addition to the analysis of synaptic convergence, the brain network diagram requires the quantitative information of divergent neuronal outputs to postsynaptic targets (Kuramoto et al. 2022). These synaptic wirings should be analyzed simultaneously with the reconstruction of their axonal morphologies, requiring a multi-scale imaging approach with various spatial—from cellular to synaptic—resolutions. CLEM is one of the anticipated potential strategies, and the technologies for imaging (Kasthuri et al. 2015; Phelps et al. 2021) and automatic dense segmentation (Turner et al. 2022) have been improved recently. CLEM with genetic fluorescence labeling and en bloc brain imaging with tissue-clearing methods that preserve ultramicrostructure (Hama et al. 2015; Furuta et al. 2022; Yamauchi et al. 2022a, 2022b) can supply the information of the exact molecular profiles of neurons to monochromatic image data from electron microscopy. Further methodological advances that systematize and semi-automate this laborious project would promote the cell type-specific description of brain-wide synaptic networks.
Although our description of synaptic dynamics in the apical tuft of L5 PT pyramidal cells offers a circuit-based mechanism for learning, there are still several problems to solve in order to reveal the whole picture of the circuit remodeling. First, we could not identify the origin of inputs to eliminated spines due to the technical limitation of post hoc immunohistochemistry, even though the spine elimination rate also increased during learning. Determination of the presynaptic characteristics of disappearing synapses requires both genetically encodable markers for cortical and thalamic inputs and in vivo imaging devices with a much higher spatial resolution than current two-photon microscopes. Second, the principle of synaptic dynamics in the perisomatic basal dendrites can differ from that in the apical tufts. The turnover of dendritic spines is active in the apical tufts of L2/3 pyramidal cells, while the basal dendrites are relatively stable during learning (Chen et al. 2015). Indeed, the apical tufts of L5 pyramidal cells have a unique property of plasticity that does not occur in the basal dendrites, exhibiting long-term synaptic potentiation induced by unpaired low-frequency stimulation (Sandler et al. 2016). The synaptic dynamics of the perisomatic basal dendrites of L5 PT cells, therefore, may adopt a different rule from those of the apical dendrites. Third, the principle of the circuit remodeling in the other cell types of M1 such as L2/3, L5 IT and L6 neurons remains to be clarified. Although L5 PT neurons are essential for motor learning (Otsuka and Kawaguchi 2021), the other cells can also contribute to learning. Indeed, learning develops the representation of the success/failure outcome in L2/3 cells that affects the activity of L5 PT cells (Levy et al. 2020). In addition, in the prelimbic cortex, L5 IT cells functionally contribute to goal-directed learning through the corticostriatal pathway (Hart et al. 2018). Thus, the complete explanation of the circuit-based mechanism in motor skill learning requires further information on presynaptic cell type-specific synaptic remodeling in all of these cell types.
Forth, L5 PT cells have several projection targets, such as the thalamus, superior colliculus and spinal cord, as well as the pontine nuclei (Oswald et al. 2013). PT cells innervate the ipsilateral pontine nuclei (Kita and Kita 2012) as they can be morphologically identified by retrograde labeling from the pontine nuclei (Hallman et al. 1988), but they are not homogeneous: for instance, upper (L5a) and lower L5 (L5b) PT neurons have a trend to project to the thalamus and the spinal cord, respectively, and a part of L5b PT cells target both of the extratelencephalic regions (Ueta et al. 2013, 2014). These divergent PT cells that command movement via projection to the spinal cord may send the efference copy to the motor thalamic nuclei that receive signals from the basal ganglia and the cerebellum; the functions of corticospinal PT neurons in motor execution and learning can depend on the presence/absence of projection to the thalamus. In particular, corticospinal neurons are located in L5b, where abundant thalamic axon fibers terminate. Therefore, corticospinal PT neurons with axon collaterals in the thalamus may form the thalamocortical loop network. The diversity of PT neurons implies the cell type-specific functions that depend on their axonal morphologies. While we did not consider the diversity of GFP-expressing L5 neurons in the Thy1-eGFP-M mouse line, the significance of PT cell outputs in motor learning would further be clarified through the observation of presynaptic cell type-dependent spine dynamics with determination of the variety of postsynaptic L5 PT neurons based on the axonal targeting.
Last, though there have been accumulated evidences that cortical GABAergic neurons are involved in synaptic dynamics and learning, the holistic picture of inhibitory microcircuit remodeling that underlies learning and memory remains elusive. The aforementioned spatially selective GABAergic networks may allow cell type-specific functions particularly in learning. GABAergic subpopulations uniquely contribute to learning and memory throughout the cerebral cortex, such as the hippocampus (McKay et al. 2013; Lovett-Barron et al. 2014; Stefanelli et al. 2016; Yap et al. 2021), the piriform (Canto-Bustos et al. 2022), motor (Chen et al. 2015; Cichon and Gan 2015; Lee et al. 2022; Ren et al. 2022; Yang et al. 2022), medial prefrontal (Cummings and Clem 2020; Cummings et al. 2022), visual (Makino and Komiyama 2015; Khan et al. 2018; Poort et al. 2022), auditory (Letzkus et al. 2011; Abs et al. 2018; Schroeder et al. 2023) and somatosensory cortices (Zhao et al. 2017). PV+, SOM+ and VIP+ neurons, three major subtypes of GABAergic neurons, uniquely contribute to synaptic plasticity (Canto-Bustos et al. 2022) and a variety of learning tasks (Khan et al. 2018; Lucas and Clem 2018; Cummings and Clem 2020; Giorgi and Marinelli 2021; Cummings et al. 2022; Lee et al. 2022; Poort et al. 2022; Ren et al. 2022; Singh and Topolnik 2023). For instance, PV+ neurons are known to participate in plasticity during the developmental critical period (Hensch 2005; Rupert and Shea 2022), and the perisomatic inhibitory subtypes such as cholecystokinin+ neurons as well as PV+ neurons in the hippocampus display opposing plasticity mechanism that may support the memory consolidation (Yap et al. 2021). On the other hand, network remodeling in learning that coincides with synaptic plasticity in pyramidal cell dendrites (Xu et al. 2009; Yang et al. 2009; Wang et al. 2011; Fu et al. 2012; Sohn et al. 2022) should be strongly affected by dendrite-targeting subpopulations such as SOM+ and L1 interneurons. Indeed, cortical SOM+ neurons have been reported to play a key role in various learning contexts (Hartung and Letzkus 2021) such as motor (Cichon and Gan 2015; Khan et al. 2018; Lee et al. 2022; Ren et al. 2022) and associative learning (McKay et al. 2013; Lovett-Barron et al. 2014; Makino and Komiyama 2015; Zhao et al. 2017; Cummings and Clem 2020; Cummings et al. 2022) with modification of structural synaptic dynamics in pyramidal cell dendrites (Chen et al. 2015; Yang et al. 2022). Interneurons in L1, where profuse top-down signals arrive, form the GABAergic microcircuit and also function in associative memory (Letzkus et al. 2011; Abs et al. 2018; Schroeder et al. 2023). Further, VIP+ neurons offer disinhibition by innervating other GABAergic cell types and, consequently, locally gate or trigger synaptic plasticity (Canto-Bustos et al. 2022) particularly in the initial learning phase (Ren et al. 2022). Thus, GABAergic neuron subclasses uniquely constitute neuronal ensembles that encode memory engrams through learning (Stefanelli et al. 2016; Giorgi and Marinelli 2021; Cummings et al. 2022).
GABA synthesis and transmission are critical for cortical plasticity and learning (Posluszny et al. 2015), and long-term synaptic plasticity is influenced by concomitant changes of GABAergic inputs in vitro (Artola and Singer 1987; Mott and Lewis 1991; Bear et al. 1992; Kanter and Haberly 1993; Brucato et al. 1996; Chapman et al. 1998; Grover and Yan 1999; Hsu et al. 1999; Kotak et al. 2017). Moreover, GABAergic synapses on pyramidal cell dendrites are dynamically modulated during learning in vivo as well (Chen et al. 2011, 2012; Villa et al. 2016). However, the identity of the presynaptic GABAergic subpopulation that directly affects the excitatory long-term plasticity is yet to be completely clarified. L1-targeting GABAergic neurons such as Martinotti cells and L1 interneurons can block the active postsynaptic events in the apical dendrites of neighboring pyramidal cells (Murayama et al. 2009; Palmer et al. 2012). The axonal boutons of SOM+ Martinotti cells are rapidly eliminated during motor learning (Chen et al. 2015); in addition, SOM+ neuron activation destabilizes dendritic spines in the apical tufts of pyramidal cells. In fact, activity of neuronal PAS domain protein 4 (NPAS4)-expressing SOM+ neurons during motor learning regulates synaptic remodeling in pyramidal cells of M1 (Yang et al. 2022). Understanding the cell type-specific contribution of GABAergic neurons, such as SOM+ Martinotti cells and L1 interneurons, to circuit remodeling during learning would require not only functional but also spatial, anatomical evidences that these GABAergic cell types in fact innervate the dendrites adjacent to the excitatory synapses with long-term plasticity. Addressing these remaining issues will be conceptually transformative and groundbreaking for a new perspective on the dynamic architecture of neural networks and the mechanism of learning and memory.
Conclusion
In this review, the author focuses on the spatial and temporal selectivity of cortical synaptic wirings that depends on the characteristics of presynaptic and postsynaptic neurons. The description of wiring diagrams at a subcellular spatial resolution requires cell characterization and tool developments including gene engineering and high-resolution microscopes combined with image processing. These technical advances visualize the spatially selective synaptic configuration in the somatodendritic structure of single neurons as well as laminar and columnar spreads of dendrites and axons. Furthermore, in vivo observation of postsynaptic dynamics combined with presynaptic cell identification unveils the principles of synaptic reconfiguration, indicating circuit-specific function in learning. Extending and integrating our knowledge of the spatial configuration and temporal reconfiguration of cortical networks will shed light on how the neocortex works in various functions. Thus, observation of synaptic dynamics based on the subcellular “connectome” may enable us to depict “functional connectome” that underlies multimodal information processing in the neocortex.
Data availability
Not applicable.
References
Abbas AI, Sundiang MJM, Henoch B et al (2018) Somatostatin interneurons facilitate hippocampal-prefrontal synchrony and prefrontal spatial encoding. Neuron 100:926-939.e923
Abs E, Poorthuis RB, Apelblat D et al (2018) Learning-related plasticity in dendrite-targeting layer 1 interneurons. Neuron 100:684-699.e686
Adler A, Zhao R, Shin ME, Yasuda R, Gan WB (2019) Somatostatin-expressing interneurons enable and maintain learning-dependent sequential activation of pyramidal neurons. Neuron 102:202-216.e207
Allen WE, Kauvar IV, Chen MZ et al (2017) Global representations of goal-directed behavior in distinct cell types of mouse neocortex. Neuron 94:891-907.e896
Altman-Hamamdzic S, Groseclose C, Ma JX et al (1997) Expression of beta-galactosidase in mouse brain: utilization of a novel nonreplicative Sindbis virus vector as a neuronal gene delivery system. Gene Ther 4:815–822
Amendola M, Venneri MA, Biffi A, Vigna E, Naldini L (2005) Coordinate dual-gene transgenesis by lentiviral vectors carrying synthetic bidirectional promoters. Nat Biotechnol 23:108–116
Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD (2005) Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron 45:207–221
Arnold PB, Li CX, Waters RS (2001) Thalamocortical arbors extend beyond single cortical barrels: an in vivo intracellular tracing study in rat. Exp Brain Res 136:152–168
Artola A, Singer W (1987) Long-term potentiation and NMDA receptors in rat visual cortex. Nature 330:649–652
Ascoli GA, Alonso-Nanclares L, Anderson SA et al (2008) Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci 9:557–568
Bear MF, Press WA, Connors BW (1992) Long-term potentiation in slices of kitten visual cortex and the effects of NMDA receptor blockade. J Neurophysiol 67:841–851
Beier KT (2019) Hitchhiking on the neuronal highway: mechanisms of transsynaptic specificity. J Chem Neuroanat 99:9–17
Brucato FH, Levin ED, Mott DD, Lewis DV, Wilson WA, Swartzwelder HS (1996) Hippocampal long-term potentiation and spatial learning in the rat: effects of GABAB receptor blockade. Neuroscience 74:331–339
Callaway EM (2005) A molecular and genetic arsenal for systems neuroscience. Trends Neurosci 28:196–201
Canto-Bustos M, Friason FK, Bassi C, Oswald AM (2022) Disinhibitory circuitry gates associative synaptic plasticity in olfactory cortex. J Neurosci 42:2942–2950
Cao VY, Ye Y, Mastwal S et al (2015) Motor learning consolidates arc-expressing neuronal ensembles in secondary motor cortex. Neuron 86:1385–1392
Casello SM, Flores RJ, Yarur HE et al (2022) Neuropeptide system regulation of prefrontal cortex circuitry: implications for neuropsychiatric disorders. Front Neural Circuits 16:796443
Challis RC, Ravindra Kumar S, Chen X et al (2022) Adeno-associated virus toolkit to target diverse brain cells. Annu Rev Neurosci 45:447–469
Chapman CA, Perez Y, Lacaille JC (1998) Effects of GABA(A) inhibition on the expression of long-term potentiation in CA1 pyramidal cells are dependent on tetanization parameters. Hippocampus 8:289–298
Chen JL, Lin WC, Cha JW, So PT, Kubota Y, Nedivi E (2011) Structural basis for the role of inhibition in facilitating adult brain plasticity. Nat Neurosci 14:587–594
Chen JL, Villa KL, Cha JW, So PT, Kubota Y, Nedivi E (2012) Clustered dynamics of inhibitory synapses and dendritic spines in the adult neocortex. Neuron 74:361–373
Chen SX, Kim AN, Peters AJ, Komiyama T (2015) Subtype-specific plasticity of inhibitory circuits in motor cortex during motor learning. Nat Neurosci 18:1109–1115
Cichon J, Gan WB (2015) Branch-specific dendritic Ca(2+) spikes cause persistent synaptic plasticity. Nature 520:180–185
Cowan RL, Wilson CJ (1994) Spontaneous firing patterns and axonal projections of single corticostriatal neurons in the rat medial agranular cortex. J Neurophysiol 71:17–32
Cronin J, Zhang XY, Reiser J (2005) Altering the tropism of lentiviral vectors through pseudotyping. Curr Gene Ther 5:387–398
Cummings KA, Clem RL (2020) Prefrontal somatostatin interneurons encode fear memory. Nat Neurosci 23:61–74
Cummings KA, Bayshtok S, Dong TN, Kenny PJ, Clem RL (2022) Control of fear by discrete prefrontal GABAergic populations encoding valence-specific information. Neuron 110:3036-3052.e3035
Daigle TL, Madisen L, Hage TA et al (2018) A suite of transgenic driver and reporter mouse lines with enhanced brain-cell-type targeting and functionality. Cell 174:465-480.e422
Davidson BL, Breakefield XO (2003) Viral vectors for gene delivery to the nervous system. Nat Rev Neurosci 4:353–364
Economo MN, Viswanathan S, Tasic B et al (2018) Distinct descending motor cortex pathways and their roles in movement. Nature 563:79–84
Feldmeyer D, Qi G, Emmenegger V, Staiger JF (2018) Inhibitory interneurons and their circuit motifs in the many layers of the barrel cortex. Neuroscience 368:132–151
Feng G, Mellor RH, Bernstein M et al (2000) Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28:41–51
Fu M, Yu X, Lu J, Zuo Y (2012) Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature 483:92–95
Fu Y, Tucciarone JM, Espinosa JS et al (2014) A cortical circuit for gain control by behavioral state. Cell 156:1139–1152
Furuta T, Tomioka R, Taki K, Nakamura K, Tamamaki N, Kaneko T (2001) In vivo transduction of central neurons using recombinant Sindbis virus: Golgi-like labeling of dendrites and axons with membrane-targeted fluorescent proteins. J Histochem Cytochem 49:1497–1508
Furuta T, Kaneko T, Deschênes M (2009) Septal neurons in barrel cortex derive their receptive field input from the lemniscal pathway. J Neurosci 29:4089–4095
Furuta T, Deschênes M, Kaneko T (2011) Anisotropic distribution of thalamocortical boutons in barrels. J Neurosci 31:6432–6439
Furuta T, Yamauchi K, Okamoto S et al (2022) Multi-scale light microscopy/electron microscopy neuronal imaging from brain to synapse with a tissue clearing method. ScaleSF Iscience 25:103601
Gentet LJ, Kremer Y, Taniguchi H, Huang ZJ, Staiger JF, Petersen CC (2012) Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nat Neurosci 15:607–612
Gerfen CR, Paletzki R, Heintz N (2013) GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron 80:1368–1383
Giorgi C, Marinelli S (2021) Roles and transcriptional responses of inhibitory neurons in learning and memory. Front Mol Neurosci 14:689952
Goldberg JH, Tamas G, Yuste R (2003) Ca2+ imaging of mouse neocortical interneurone dendrites: Ia-type K+ channels control action potential backpropagation. J Physiol 551:49–65
Gonchar Y, Burkhalter A (1997) Three distinct families of GABAergic neurons in rat visual cortex. Cereb Cortex 7:347–358
Gouwens NW, Sorensen SA, Baftizadeh F et al (2020) Integrated morphoelectric and transcriptomic classification of cortical GABAergic cells. Cell 183:935-953.e919
Grover LM, Yan C (1999) Blockade of GABAA receptors facilitates induction of NMDA receptor-independent long-term potentiation. J Neurophysiol 81:2814–2822
Grutzendler J, Kasthuri N, Gan WB (2002) Long-term dendritic spine stability in the adult cortex. Nature 420:812–816
Guo ZV, Li N, Huber D et al (2014) Flow of cortical activity underlying a tactile decision in mice. Neuron 81:179–194
Gwag BJ, Kim EY, Ryu BR et al (1998) A neuron-specific gene transfer by a recombinant defective Sindbis virus. Brain Res Mol Brain Res 63:53–61
Hajós F, Zilles K (1988) Quantitative immunohistochemical analysis of VIP-neurons in the rat visual cortex. Histochemistry 90:139–144
Hallman LE, Schofield BR, Lin CS (1988) Dendritic morphology and axon collaterals of corticotectal, corticopontine, and callosal neurons in layer V of primary visual cortex of the hooded rat. J Comp Neurol 272:149–160
Hama H, Hioki H, Namiki K et al (2015) ScaleS: an optical clearing palette for biological imaging. Nat Neurosci 18:1518–1529
Han Y, Kebschull JM, Campbell RAA et al (2018) The logic of single-cell projections from visual cortex. Nature 556:51–56
Hart G, Bradfield LA, Fok SY, Chieng B, Balleine BW (2018) The bilateral prefronto-striatal pathway is necessary for learning new goal-directed actions. Curr Biol 28:2218-2229.e2217
Hartung J, Letzkus JJ (2021) Inhibitory plasticity in layer 1—dynamic gatekeeper of neocortical associations. Curr Opin Neurobiol 67:26–33
Hasegawa R, Ebina T, Tanaka YR, Kobayashi K, Matsuzaki M (2020) Structural dynamics and stability of corticocortical and thalamocortical axon terminals during motor learning. PLoS ONE 15:e0234930
Hayashi-Takagi A, Yagishita S, Nakamura M et al (2015) Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature 525:333–338
He M, Tucciarone J, Lee S et al (2016) Strategies and tools for combinatorial targeting of GABAergic neurons in mouse cerebral cortex. Neuron 91:1228–1243
Hensch TK (2005) Critical period plasticity in local cortical circuits. Nat Rev Neurosci 6:877–888
Hilscher MM, Leão RN, Edwards SJ, Leão KE, Kullander K (2017) Chrna2-martinotti cells synchronize layer 5 type a pyramidal cells via rebound excitation. PLoS Biol 15:e2001392
Hioki H, Kameda H, Nakamura H et al (2007) Efficient gene transduction of neurons by lentivirus with enhanced neuron-specific promoters. Gene Ther 14:872–882
Hioki H, Kuramoto E, Konno M et al (2009) High-level transgene expression in neurons by lentivirus with tet-off system. Neurosci Res 63:149–154
Hioki H, Okamoto S, Konno M et al (2013) Cell type-specific inhibitory inputs to dendritic and somatic compartments of parvalbumin-expressing neocortical interneuron. J Neurosci 33:544–555
Hioki H, Sohn J, Nakamura H et al (2018) Preferential inputs from cholecystokinin-positive neurons to the somatic compartment of parvalbumin-expressing neurons in the mouse primary somatosensory cortex. Brain Res 1695:18–30
Hirai Y, Morishima M, Karube F, Kawaguchi Y (2012) Specialized cortical subnetworks differentially connect frontal cortex to parahippocampal areas. J Neurosci 32:1898–1913
Hirano M, Kato S, Kobayashi K, Okada T, Yaginuma H, Kobayashi K (2013) Highly efficient retrograde gene transfer into motor neurons by a lentiviral vector pseudotyped with fusion glycoprotein. PLoS ONE 8:e75896
Hodge RD, Bakken TE, Miller JA et al (2019) Conserved cell types with divergent features in human versus mouse cortex. Nature 573:61–68
Hooks BM, Mao T, Gutnisky DA, Yamawaki N, Svoboda K, Shepherd GM (2013) Organization of cortical and thalamic input to pyramidal neurons in mouse motor cortex. J Neurosci 33:748–760
Hostetler RE, Hu H, Agmon A (2023) Genetically defined subtypes of somatostatin-containing cortical interneurons. eNeuro 10:ENEURO.0204–23.2023
Hsu KS, Ho WC, Huang CC, Tsai JJ (1999) Prior short-term synaptic disinhibition facilitates long-term potentiation and suppresses long-term depression at CA1 hippocampal synapses. Eur J Neurosci 11:4059–4069
Im S, Ueta Y, Otsuka T et al (2023) Corticocortical innervation subtypes of layer 5 intratelencephalic cells in the murine secondary motor cortex. Cereb Cortex 33:50–67
Iwano S, Sugiyama M, Hama H et al (2018) Single-cell bioluminescence imaging of deep tissue in freely moving animals. Science 359:935–939
Jiang X, Wang G, Lee AJ, Stornetta RL, Zhu JJ (2013) The organization of two new cortical interneuronal circuits. Nat Neurosci 16:210–218
Jiang X, Shen S, Cadwell CR et al. (2015) Principles of connectivity among morphologically defined cell types in adult neocortex. Science 350:aac9462
Jones-Tabah J, Mohammad H, Hadj-Youssef S et al (2020) Dopamine D1 receptor signalling in dyskinetic Parkinsonian rats revealed by fiber photometry using FRET-based biosensors. Sci Rep 10:14426
Junyent F, Kremer EJ (2015) CAV-2–why a canine virus is a neurobiologist’s best friend. Curr Opin Pharmacol 24:86–93
Kameda H, Hioki H, Tanaka YH et al (2012) Parvalbumin-producing cortical interneurons receive inhibitory inputs on proximal portions and cortical excitatory inputs on distal dendrites. Eur J Neurosci 35:838–854
Kamigaki T, Dan Y (2017) Delay activity of specific prefrontal interneuron subtypes modulates memory-guided behavior. Nat Neurosci 20:854–863
Kaneko T (2013) Local connections of excitatory neurons in motor-associated cortical areas of the rat. Front Neural Circuits 7:75
Kaneko R, Takatsuru Y, Morita A et al (2018) Inhibitory neuron-specific Cre-dependent red fluorescent labeling using VGAT BAC-based transgenic mouse lines with identified transgene integration sites. J Comp Neurol 526:373–396
Kanter ED, Haberly LB (1993) Associative long-term potentiation in piriform cortex slices requires GABAA blockade. J Neurosci 13:2477–2482
Kaplitt MG, Leone P, Samulski RJ et al (1994) Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet 8:148–154
Karnani MM, Jackson J, Ayzenshtat I et al (2016) Cooperative subnetworks of molecularly similar interneurons in mouse neocortex. Neuron 90:86–100
Kasthuri N, Hayworth KJ, Berger DR et al (2015) Saturated reconstruction of a volume of neocortex. Cell 162:648–661
Kato S, Kobayashi K (2020) Pseudotyped lentiviral vectors for tract-targeting and application for the functional control of selective neural circuits. J Neurosci Methods 344:108854
Kawaguchi Y (2017) Pyramidal cell subtypes and their synaptic connections in layer 5 of rat frontal cortex. Cereb Cortex 27:5755–5771
Kawaguchi Y, Kubota Y (1996) Physiological and morphological identification of somatostatin- or vasoactive intestinal polypeptide-containing cells among GABAergic cell subtypes in rat frontal cortex. J Neurosci 16:2701–2715
Kawaguchi Y, Kubota Y (1997) GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex 7:476–486
Kelly RM, Strick PL (2000) Rabies as a transneuronal tracer of circuits in the central nervous system. J Neurosci Methods 103:63–71
Kepecs A, Fishell G (2014) Interneuron cell types are fit to function. Nature 505:318–326
Khan AG, Poort J, Chadwick A et al (2018) Distinct learning-induced changes in stimulus selectivity and interactions of GABAergic interneuron classes in visual cortex. Nat Neurosci 21:851–859
Kita T, Kita H (2012) The subthalamic nucleus is one of multiple innervation sites for long-range corticofugal axons: a single-axon tracing study in the rat. J Neurosci 32:5990–5999
Kotak VC, Mirallave A, Mowery TM, Sanes DH (2017) GABAergic inhibition gates excitatory LTP in perirhinal cortex. Hippocampus 27:1217–1223
Kubota Y (2014) Untangling GABAergic wiring in the cortical microcircuit. Curr Opin Neurobiol 26:7–14
Kubota Y, Hattori R, Yui Y (1994) Three distinct subpopulations of GABAergic neurons in rat frontal agranular cortex. Brain Res 649:159–173
Kubota Y, Shigematsu N, Karube F et al (2011) Selective coexpression of multiple chemical markers defines discrete populations of neocortical GABAergic neurons. Cereb Cortex 21:1803–1817
Kubota Y, Karube F, Nomura M, Kawaguchi Y (2016) The diversity of cortical inhibitory synapses. Front Neural Circuits 10:27
Kubota Y, Kondo S, Nomura M et al. (2015) Functional effects of distinct innervation styles of pyramidal cells by fast spiking cortical interneurons. Elife 4
Kuramoto E, Furuta T, Nakamura KC, Unzai T, Hioki H, Kaneko T (2009) Two types of thalamocortical projections from the motor thalamic nuclei of the rat: a single neuron-tracing study using viral vectors. Cereb Cortex 19:2065–2077
Kuramoto E, Fujiyama F, Nakamura KC, Tanaka Y, Hioki H, Kaneko T (2011) Complementary distribution of glutamatergic cerebellar and GABAergic basal ganglia afferents to the rat motor thalamic nuclei. Eur J Neurosci 33:95–109
Kuramoto E, Ohno S, Furuta T et al (2015) Ventral medial nucleus neurons send thalamocortical afferents more widely and more preferentially to layer 1 than neurons of the ventral anterior-ventral lateral nuclear complex in the rat. Cereb Cortex 25:221–235
Kuramoto E, Pan S, Furuta T et al (2017) Individual mediodorsal thalamic neurons project to multiple areas of the rat prefrontal cortex: a single neuron-tracing study using virus vectors. J Comp Neurol 525:166–185
Kuramoto E, Tanaka YR, Hioki H, Goto T, Kaneko T (2022) Local connections of pyramidal neurons to parvalbumin-producing interneurons in motor-associated cortical areas of mice. eNeuro 9
Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B (2010) The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci 30:16796–16808
Lee SH, Kwan AC, Zhang S et al (2012) Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature 488:379–383
Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy B (2013) A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci 16:1662–1670
Lee AJ, Wang G, Jiang X et al (2015) Canonical organization of layer 1 neuron-led cortical inhibitory and disinhibitory interneuronal circuits. Cereb Cortex 25:2114–2126
Lee AT, Cunniff MM, See JZ et al (2019) VIP interneurons contribute to avoidance behavior by regulating information flow across hippocampal-prefrontal networks. Neuron 102:1223-1234.e1224
Lee C, Harkin EF, Yin X, Naud R, Chen S (2022) Cell-type-specific responses to associative learning in the primary motor cortex. Elife 11
Letzkus JJ, Wolff SB, Meyer EM et al (2011) A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 480:331–335
Levy S, Lavzin M, Benisty H et al (2020) Cell-type-specific outcome representation in the primary motor cortex. Neuron 107:954-971.e959
Li N, Chen TW, Guo ZV, Gerfen CR, Svoboda K (2015) A motor cortex circuit for motor planning and movement. Nature 519:51–56
Little JP, Carter AG (2012) Subcellular synaptic connectivity of layer 2 pyramidal neurons in the medial prefrontal cortex. J Neurosci 32:12808–12819
Liu X, Carter AG (2018) Ventral hippocampal inputs preferentially drive corticocortical neurons in the infralimbic prefrontal cortex. J Neurosci 38:7351–7363
Livet J, Weissman TA, Kang H et al (2007) Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450:56–62
Loh R, Collins S, Galvez R (2017) Neocortical prodynorphin expression is transiently increased with learning: Implications for time- and learning-dependent neocortical kappa opioid receptor activation. Behav Brain Res 335:145–150
Lorente de Nó R (1938) Cerebral cortex: architecture, intracortical connections, motor projections. In: Fulton JF (ed) Physiology of Nervous System, 2nd edn. Oxford University Press, London, pp 291–339
Lovett-Barron M, Turi GF, Kaifosh P et al (2012) Regulation of neuronal input transformations by tunable dendritic inhibition. Nat Neurosci 15(423–430):s421-423
Lovett-Barron M, Kaifosh P, Kheirbek MA et al (2014) Dendritic inhibition in the hippocampus supports fear learning. Science 343:857–863
Lu J, Tucciarone J, Padilla-Coreano N, He M, Gordon JA, Huang ZJ (2017) Selective inhibitory control of pyramidal neuron ensembles and cortical subnetworks by chandelier cells. Nat Neurosci 20:1377–1383
Lucas EK, Clem RL (2018) GABAergic interneurons: The orchestra or the conductor in fear learning and memory? Brain Res Bull 141:13–19
Lukashchuk V, Lewis KE, Coldicott I, Grierson AJ, Azzouz M (2016) AAV9-mediated central nervous system-targeted gene delivery via cisterna magna route in mice. Mol Ther Methods Clin Dev 3:15055
Ma Y, Hu H, Berrebi AS, Mathers PH, Agmon A (2006) Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J Neurosci 26:5069–5082
Madisen L, Zwingman TA, Sunkin SM et al (2010) A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13:133–140
Makino H, Komiyama T (2015) Learning enhances the relative impact of top-down processing in the visual cortex. Nat Neurosci 18:1116–1122
Makino H, Ren C, Liu H et al (2017) Transformation of cortex-wide emergent properties during motor learning. Neuron 94:880-890.e888
Manita S, Suzuki T, Homma C et al (2015) A top-down cortical circuit for accurate sensory perception. Neuron 86:1304–1316
Mao T, Kusefoglu D, Hooks BM, Huber D, Petreanu L, Svoboda K (2011) Long-range neuronal circuits underlying the interaction between sensory and motor cortex. Neuron 72:111–123
Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C (2004) Interneurons of the neocortical inhibitory system. Nat Rev Neurosci 5:793–807
McKay BM, Oh MM, Disterhoft JF (2013) Learning increases intrinsic excitability of hippocampal interneurons. J Neurosci 33:5499–5506
Meng JH, Schuman B, Rudy B, Wang XJ (2023) Mechanisms of Dominant Electrophysiological Features of Four Subtypes of Layer 1 Interneurons. J Neurosci
Miyoshi H, Blömer U, Takahashi M, Gage FH, Verma IM (1998) Development of a self-inactivating lentivirus vector. J Virol 72:8150–8157
Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD (2007) Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci 8:427–437
Morishima M, Kawaguchi Y (2006) Recurrent connection patterns of corticostriatal pyramidal cells in frontal cortex. J Neurosci 26:4394–4405
Morishima M, Morita K, Kubota Y, Kawaguchi Y (2011) Highly differentiated projection-specific cortical subnetworks. J Neurosci 31:10380–10391
Moriyoshi K, Richards LJ, Akazawa C, O’Leary DD, Nakanishi S (1996) Labeling neural cells using adenoviral gene transfer of membrane-targeted GFP. Neuron 16:255–260
Mott DD, Lewis DV (1991) Facilitation of the induction of long-term potentiation by GABAB receptors. Science 252:1718–1720
Muñoz W, Tremblay R, Levenstein D, Rudy B (2017) Layer-specific modulation of neocortical dendritic inhibition during active wakefulness. Science 355:954–959
Murayama M, Pérez-Garci E, Nevian T, Bock T, Senn W, Larkum ME (2009) Dendritic encoding of sensory stimuli controlled by deep cortical interneurons. Nature 457:1137–1141
Nakamura H, Hioki H, Furuta T, Kaneko T (2015) Different cortical projections from three subdivisions of the rat lateral posterior thalamic nucleus: a single-neuron tracing study with viral vectors. Eur J Neurosci 41:1294–1310
Naldini L, Blömer U, Gallay P et al (1996) In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272:263–267
Nigro MJ, Hashikawa-Yamasaki Y, Rudy B (2018) Diversity and connectivity of layer 5 somatostatin-expressing interneurons in the mouse barrel cortex. J Neurosci 38:1622–1633
Niwa H, Yamamura K, Miyazaki J (1991) Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193–199
Ohno S, Kuramoto E, Furuta T et al (2012) A morphological analysis of thalamocortical axon fibers of rat posterior thalamic nuclei: a single neuron tracing study with viral vectors. Cereb Cortex 22:2840–2857
Oswald MJ, Tantirigama ML, Sonntag I, Hughes SM, Empson RM (2013) Diversity of layer 5 projection neurons in the mouse motor cortex. Front Cell Neurosci 7:174
Otsuka T, Kawaguchi Y (2021) Pyramidal cell subtype-dependent cortical oscillatory activity regulates motor learning. Commun Biol 4:495
Palmer LM, Schulz JM, Murphy SC, Ledergerber D, Murayama M, Larkum ME (2012) The cellular basis of GABA(B)-mediated interhemispheric inhibition. Science 335:989–993
Peng H, Xie P, Liu L et al (2021) Morphological diversity of single neurons in molecularly defined cell types. Nature 598:174–181
Peters AJ, Chen SX, Komiyama T (2014) Emergence of reproducible spatiotemporal activity during motor learning. Nature 510:263–267
Petreanu L, Huber D, Sobczyk A, Svoboda K (2007) Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci 10:663–668
Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M (2013) Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci 16:1068–1076
Phelps JS, Hildebrand DGC, Graham BJ et al (2021) Reconstruction of motor control circuits in adult Drosophila using automated transmission electron microscopy. Cell 184:759-774.e718
Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A (2013) Cortical interneurons that specialize in disinhibitory control. Nature 503:521–524
Poort J, Wilmes KA, Blot A et al (2022) Learning and attention increase visual response selectivity through distinct mechanisms. Neuron 110:686-697.e686
Porter JT, Cauli B, Staiger JF, Lambolez B, Rossier J, Audinat E (1998) Properties of bipolar VIPergic interneurons and their excitation by pyramidal neurons in the rat neocortex. Eur J Neurosci 10:3617–3628
Posluszny A, Liguz-Lecznar M, Turzynska D, Zakrzewska R, Bielecki M, Kossut M (2015) Learning-dependent plasticity of the barrel cortex is impaired by restricting GABA-Ergic transmission. PLoS ONE 10:e0144415
Reiner A, Jiao Y, Del Mar N, Laverghetta AV, Lei WL (2003) Differential morphology of pyramidal tract-type and intratelencephalically projecting-type corticostriatal neurons and their intrastriatal terminals in rats. J Comp Neurol 457:420–440
Ren C, Peng K, Yang R, Liu W, Liu C, Komiyama T (2022) Global and subtype-specific modulation of cortical inhibitory neurons regulated by acetylcholine during motor learning. Neuron 110:2334-2350.e2338
Rockland KS (2020) What we can learn from the complex architecture of single axons. Brain Struct Funct 225:1327–1347
Rozov A, Jerecic J, Sakmann B, Burnashev N (2001) AMPA receptor channels with long-lasting desensitization in bipolar interneurons contribute to synaptic depression in a novel feedback circuit in layer 2/3 of rat neocortex. J Neurosci 21:8062–8071
Rudy B, Fishell G, Lee S, Hjerling-Leffler J (2011) Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol 71:45–61
Rupert DD, Shea SD (2022) Parvalbumin-positive interneurons regulate cortical sensory plasticity in adulthood and development through shared mechanisms. Front Neural Circuits 16:886629
Ry C (1911) Histologie du système nerveux de l’homme & des vertébrés. Maloine, Paris
Saito T, Nakatsuji N (2001) Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev Biol 240:237–246
Sandler M, Shulman Y, Schiller J (2016) A novel form of local plasticity in tuft dendrites of neocortical somatosensory layer 5 pyramidal neurons. Neuron 90:1028–1042
Schroeder A, Pardi MB, Keijser J et al (2023) Inhibitory top-down projections from zona incerta mediate neocortical memory. Neuron 111:727-738.e728
Schuman B, Machold RP, Hashikawa Y, Fuzik J, Fishell GJ, Rudy B (2019) Four unique interneuron populations reside in neocortical layer 1. J Neurosci 39:125–139
Shigematsu N, Ueta Y, Mohamed AA et al (2016) Selective thalamic innervation of rat frontal cortical neurons. Cereb Cortex 26:2689–2704
Singh S, Topolnik L (2023) Inhibitory circuits in fear memory and fear-related disorders. Front Neural Circuits 17:1122314
Smith SJ, Sümbül U, Graybuck LT et al. (2019) Single-cell transcriptomic evidence for dense intracortical neuropeptide networks. Elife 8
Sohn J, Hioki H, Okamoto S, Kaneko T (2014) Preprodynorphin-expressing neurons constitute a large subgroup of somatostatin-expressing GABAergic interneurons in the mouse neocortex. J Comp Neurol 522:1506–1526
Sohn J, Okamoto S, Kataoka N, Kaneko T, Nakamura K, Hioki H (2016) Differential inputs to the perisomatic and distal-dendritic compartments of VIP-positive neurons in layer 2/3 of the mouse barrel cortex. Front Neuroanat 10:124
Sohn J, Takahashi M, Okamoto S, Ishida Y, Furuta T, Hioki H (2017) A single vector platform for high-level gene transduction of central neurons: adeno-associated virus vector equipped with the tet-off system. PLoS ONE 12:e0169611
Sohn J, Suzuki M, Youssef M et al. (2022) Presynaptic supervision of cortical spine dynamics in motor learning. Sci Adv 8:eabm0531
Somogyi P, Tamás G, Lujan R, Buhl EH (1998) Salient features of synaptic organisation in the cerebral cortex. Brain Res Brain Res Rev 26:113–135
Soudais C, Laplace-Builhe C, Kissa K, Kremer EJ (2001) Preferential transduction of neurons by canine adenovirus vectors and their efficient retrograde transport in vivo. Faseb j 15:2283–2285
Staiger JF, Zilles K, Freund TF (1996) Innervation of VIP-immunoreactive neurons by the ventroposteromedial thalamic nucleus in the barrel cortex of the rat. J Comp Neurol 367:194–204
Staiger JF, Freund TF, Zilles K (1997) Interneurons immunoreactive for vasoactive intestinal polypeptide (VIP) are extensively innervated by parvalbumin-containing boutons in rat primary somatosensory cortex. Eur J Neurosci 9:2259–2268
Staiger JF, Schubert D, Zuschratter W, Kötter R, Luhmann HJ, Zilles K (2002) Innervation of interneurons immunoreactive for VIP by intrinsically bursting pyramidal cells and fast-spiking interneurons in infragranular layers of juvenile rat neocortex. Eur J Neurosci 16:11–20
Stefanelli T, Bertollini C, Lüscher C, Muller D, Mendez P (2016) Hippocampal somatostatin interneurons control the size of neuronal memory ensembles. Neuron 89:1074–1085
Sun YC, Chen X, Fischer S et al (2021) Integrating barcoded neuroanatomy with spatial transcriptional profiling enables identification of gene correlates of projections. Nat Neurosci 24:873–885
Tabata H, Nakajima K (2001) Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscience 103:865–872
Tamamaki N, Nakamura K, Furuta T, Asamoto K, Kaneko T (2000) Neurons in Golgi-stain-like images revealed by GFP-adenovirus infection in vivo. Neurosci Res 38:231–236
Taniguchi H, He M, Wu P et al (2011) A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71:995–1013
Tasic B, Menon V, Nguyen TN et al (2016) Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat Neurosci 19:335–346
Tervo DG, Hwang BY, Viswanathan S et al (2016) A designer AAV variant permits efficient retrograde access to projection neurons. Neuron 92:372–382
Thomson AM, Bannister AP (2003) Interlaminar connections in the neocortex. Cereb Cortex 13:5–14
Tomioka R, Rockland KS (2006) Improved Golgi-like visualization in retrogradely projecting neurons after EGFP-adenovirus infection in adult rat and monkey. J Histochem Cytochem 54:539–548
Trachtenberg JT, Chen BE, Knott GW et al (2002) Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature 420:788–794
Tremblay R, Lee S, Rudy B (2016) GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron 91:260–292
Turner NL, Macrina T, Bae JA et al (2022) Reconstruction of neocortex: organelles, compartments, cells, circuits, and activity. Cell 185:1082-1100.e1024
Uematsu M, Hirai Y, Karube F et al (2008) Quantitative chemical composition of cortical GABAergic neurons revealed in transgenic venus-expressing rats. Cereb Cortex 18:315–330
Ueta Y, Hirai Y, Otsuka T, Kawaguchi Y (2013) Direction- and distance-dependent interareal connectivity of pyramidal cell subpopulations in the rat frontal cortex. Front Neural Circuits 7:164
Ueta Y, Otsuka T, Morishima M, Ushimaru M, Kawaguchi Y (2014) Multiple layer 5 pyramidal cell subtypes relay cortical feedback from secondary to primary motor areas in rats. Cereb Cortex 24:2362–2376
Ueta Y, Sohn J, Agahari FA et al (2019) Ipsi- and contralateral corticocortical projection-dependent subcircuits in layer 2 of the rat frontal cortex. J Neurophysiol 122:1461–1472
van Aerde KI, Feldmeyer D (2015) Morphological and physiological characterization of pyramidal neuron subtypes in rat medial prefrontal cortex. Cereb Cortex 25:788–805
van den Pol AN, Ozduman K, Wollmann G et al (2009) Viral strategies for studying the brain, including a replication-restricted self-amplifying delta-G vesicular stomatis virus that rapidly expresses transgenes in brain and can generate a multicolor golgi-like expression. J Comp Neurol 516:456–481
Veinante P, Deschênes M (2003) Single-cell study of motor cortex projections to the barrel field in rats. J Comp Neurol 464:98–103
Villa KL, Berry KP, Subramanian J et al (2016) Inhibitory synapses are repeatedly assembled and removed at persistent sites in vivo. Neuron 89:756–769
Wang L, Conner JM, Rickert J, Tuszynski MH (2011) Structural plasticity within highly specific neuronal populations identifies a unique parcellation of motor learning in the adult brain. Proc Natl Acad Sci U S A 108:2545–2550
Wilson NR, Runyan CA, Wang FL, Sur M (2012) Division and subtraction by distinct cortical inhibitory networks in vivo. Nature 488:343–348
Wolff SBE, Ko R, Ölveczky BP (2022) Distinct roles for motor cortical and thalamic inputs to striatum during motor skill learning and execution. Sci Adv 8:eabk0231
Xu X, Roby KD, Callaway EM (2006) Mouse cortical inhibitory neuron type that coexpresses somatostatin and calretinin. J Comp Neurol 499:144–160
Xu T, Yu X, Perlik AJ et al (2009) Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 462:915–919
Xu X, Roby KD, Callaway EM (2010) Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J Comp Neurol 518:389–404
Xu H, Liu L, Tian Y et al (2019) A disinhibitory microcircuit mediates conditioned social fear in the prefrontal cortex. Neuron 102:668-682.e665
Xu Z, Geron E, Pérez-Cuesta LM, Bai Y, Gan WB (2023) Generalized extinction of fear memory depends on co-allocation of synaptic plasticity in dendrites. Nat Commun 14:503
Yamashita T, Pala A, Pedrido L, Kremer Y, Welker E, Petersen CC (2013) Membrane potential dynamics of neocortical projection neurons driving target-specific signals. Neuron 80:1477–1490
Yamauchi K, Furuta T, Okamoto S, Takahashi M, Koike M, Hioki H (2022a) Protocol for multi-scale light microscopy/electron microscopy neuronal imaging in mouse brain tissue. STAR Protoc 3:101508
Yamauchi K, Okamoto S, Takahashi M, Koike M, Furuta T, Hioki H (2022b) A Tissue Clearing Method for Neuronal Imaging from Mesoscopic to Microscopic Scales. J Vis Exp
Yang G, Pan F, Gan WB (2009) Stably maintained dendritic spines are associated with lifelong memories. Nature 462:920–924
Yang G, Lai CS, Cichon J, Ma L, Li W, Gan WB (2014) Sleep promotes branch-specific formation of dendritic spines after learning. Science 344:1173–1178
Yang J, Serrano P, Yin X, Sun X, Lin Y, Chen SX (2022) Functionally distinct NPAS4-expressing somatostatin interneuron ensembles critical for motor skill learning. Neuron 110:3339-3355.e3338
Yap EL, Pettit NL, Davis CP et al (2021) Bidirectional perisomatic inhibitory plasticity of a Fos neuronal network. Nature 590:115–121
Yu J, Hu H, Agmon A, Svoboda K (2019) Recruitment of GABAergic interneurons in the barrel cortex during active tactile behavior. Neuron 104:412-427.e414
Zeisel A, Muñoz-Manchado AB, Codeluppi S et al (2015) Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347:1138–1142
Zhang S, Xu M, Kamigaki T et al (2014) Selective attention. Long-range and local circuits for top-down modulation of visual cortex processing. Science 345:660–665
Zhang GR, Zhao H, Abdul-Muneer PM, Cao H, Li X, Geller AI (2015) Neurons can be labeled with unique hues by helper virus-free HSV-1 vectors expressing Brainbow. J Neurosci Methods 240:77–88
Zhao X, Huang L, Guo R et al (2017) Coordinated Plasticity among Glutamatergic and GABAergic Neurons and Synapses in the Barrel Cortex Is Correlated to Learning Efficiency. Front Cell Neurosci 11:221
Zhou X, Rickmann M, Hafner G, Staiger JF (2017) Subcellular targeting of VIP boutons in mouse barrel cortex is layer-dependent and not restricted to interneurons. Cereb Cortex 27:5353–5368
Zingg B, Chou XL, Zhang ZG et al (2017) AAV-mediated anterograde transsynaptic tagging: mapping corticocollicular input-defined neural pathways for defense behaviors. Neuron 93:33–47
Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D (1997) Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol 15:871–875
Acknowledgements
The author is deeply indebted to Profs. Takeshi Kaneko (Kyoto University, Kyoto, Japan), Hiroyuki Hioki (Juntendo University, Tokyo, Japan), Takahiro Furuta (Osaka University, Osaka, Japan), Yasuo Kawaguchi (Tamagawa University, Tokyo, Japan), and Yoshiyuki Kubota (National Institute for Physiological Sciences, Aichi, Japan) for their kind supports, valuable discussions and insightful comments. The author also thanks Dr. Sukeui Sohn for editorial assistance. This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 21K15200.
Funding
Open access funding provided by Osaka University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sohn, J. Synaptic configuration and reconfiguration in the neocortex are spatiotemporally selective. Anat Sci Int 99, 17–33 (2024). https://doi.org/10.1007/s12565-023-00743-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-023-00743-5