Abstract
Aquatic organisms detect chemical cues to sense the local environment, for example, to find a mate, locate food, and identify danger. Knowledge of chemical cues can be used in aquaculture, in practical applications such as controlling mating behavior to increase fertility, enhance feeding, and decrease stress; in fisheries, by catching selected species with low-cost artificial attractants; and to address maritime issues, by decreasing biofouling. Aquatic organisms also detect chemical cues related to global environmental changes, ocean acidification, and increases in ocean plastics, all of which can affect their chemosensory behaviors. Here we discuss the nature of chemical cues and chemosensory biology and ecology of aquatic organisms, and potential applications with an emphasis on sex pheromones in commercially important and well-studied animals, namely, decapod crustaceans and fish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquatic organisms use information conveyed by chemical compounds from other organisms to find and choose food, mates, and habitat, and to avoid potential dangers, including predation and infection (Kamio and Derby 2017). Since chemical compounds affect the behavior of other organisms, some organisms emit defensive compounds to deter and/or deceive predators and parasites. Studies on these chemically mediated interactions among organisms provide insights into the ecology and evolution of marine communities (Hay 2009; Zimmer et al. 2017).
The study of chemical communication provides technical insights that are applicable for fishery and aquaculture (Barki et al. 2010; Saha et al. 2019) due to the dependence of fishery on marine ecological communities and because aquaculture involves the management of artificial ecological communities in closed or semi-closed systems with selected species, including microorganisms. The identification and artificial production of chemical cues can provide tools to attract and repel organisms, a push–pull strategy (Pickett et al. 2014). Pheromones are used to manage populations of nuisance fish (Buchinger et al. 2015; Sorensen and Johnson 2016) and to catch commercially important crabs that are not attracted to food-based bait (Kamio and Derby 2010). Thus, studies on chemical communication can provide insights and tools to manage aquatic organisms.
Marine chemical ecology is a research field that studies chemically mediated interactions among organisms in an ecological context. There are excellent reviews on marine chemical ecology in benthic environments (Paul et al. 2011; Puglisi et al. 2014, 2019) and on planktonic interactions (Hay and Kubanek 2002; Poulson et al. 2009; Ianora et al. 2011; Sieg et al. 2011; Roy et al. 2013; Schwartz et al. 2016; Brown et al. 2019) that describe chemical relations between organisms, including toxicity. In this review, we summarize the chemical nature of chemical cues and how organisms emit and receive chemical cues, and chemical communication that has the potential to benefit fisheries and aquaculture, such as sex pheromones, in crustaceans and fish, and others.
Definition of cue in this paper
In this paper, chemical cues are defined as compounds that convey information. Although a signal is defined as information evolved from a cue because it benefits the emitter of the information in natural selection (Wyatt 2017), here we use the common (receiver-based) term cue to refer to any detectable feature in the environment, as each receiver can gain behavioral meaning. Thus, here the term “chemical cue” includes food odors, predator odors, pheromones, and chemicals that alarm conspecifics.
Chemical character of cues in aquatic environments
The molecular weight range of chemical cues is wide. A variety of organic molecules, concomitant with other biogenic molecules, including nitrogen, sulfur, and phosphorus compounds, are produced and consumed by marine organisms as a part of the carbon cycle. Marine organisms smell and taste these molecules to gain knowledge of their environment (Derby and Zimmer 2012; Kamio and Derby 2017). Marine organisms detect small (i.e., < 1.5 kDa) organic molecules, called metabolites (Wishart et al. 2007), to find and select food, peptides (7–11 kDa) to find mates (Painter et al. 1998; Cummins et al. 2004), proteins (approx. 30 kDa and approx. 200 kDa and its dimer) to find specific prey (Ferrier et al. 2016; Zimmer et al. 2017), and protein complexes to achieve gregarious settlement (Matsumura et al. 1998; Dreanno et al. 2007). A wide range of molecules, including sugars (Jager 1970; Weissburg and Zimmer-Faust 1991), amino acids (Yambe et al. 2006a), nucleotides (Zimmer-Faust 1993), pyrimidine bases and nucleosides (Kicklighter et al. 2007), lipids (Sakata et al. 1988), and steroids (Dulka et al. 1987), can be chemical stimuli. Thus, marine organisms can detect small metabolites ranging up to large protein complexes as chemical cues. To study this broad spectrum of molecules, collaboration between biologists, biochemists, many different types of chemists, and molecular biologists is necessary due to the many different methods needed to analyze these different types of compounds. For example, protein is a large peptide that is a polymer of amino acids; however, the isolation and identification of amino acids, peptides, and proteins, respectively, require different techniques and equipment. Amino acids are effectively quantified using an amino acid analyzer or by liquid chromatography/mass spectrometry (LC–MS). Peptides are sequenced using a peptide sequencer or fragmentation analysis in MS. Proteins are large peptides and partially sequenced in the same manner as peptides, following which the whole protein is identified by DNA sequencing using the partial sequence as a probe. The field of natural products chemistry involves the purification and identification of a broad range of small molecules using nuclear magnetic resonance (NMR), MS, and other analytical methods, and synthesis of the proposed structure. However, the purification of each compound needs different techniques and equipment depending on the polarity, volatility, acidity, molecular size, and other physicochemical characters of the compound; for example, purification of a volatile compound requires a purification system different from that for nonvolatile compounds.
A variety of information is carried by chemical compounds. Consumers eat other organisms and digest them to obtain the primary metabolites used as building blocks of their body and to obtain energy. Traditional metabolites are roughly classified into two categories: primary and secondary metabolites. Primary metabolites are common molecules and thus can be general chemical markers of the status of animals: alive, injured, or dead. Characteristic molecules that are specific to certain species and higher order taxonomic groups, or to ecological niches, are called secondary metabolites and can be chemical markers of species, communities, and/or the physiological state of the organisms releasing them. Secondary metabolites that have ecological functions can be digested to become primary metabolites; however, some of them are sequestered without modification (Pennings and Paul 1993; Kamio et al. 2011, 2016a; Kicklighter et al. 2011) or modified to take on new ecological functions (Kamio et al. 2010a, 2010b) and be used in the same and other ecological context. Proteins have complex and diverse structures based on genomic DNA sequence and thus can be specific to the organism. Organisms also use combinations of molecules as specific cues of the object in finding food, engaging in social interactions, such as courtship, competition, and aggregation, and avoiding predators. Variations in distant cues in the aquatic environment can be more diverse than those in the terrestrial environment.
How chemical cues are released from organisms
Chemicals are stored in an organism’s body and are released from the body to be detected by other organisms. There are several ways for this release to occur. One way is through injury. Dissolved free amino acids (DFAAs), which are the most common appetitive metabolites that stimulate food searching in lobsters and fish, can be found at high concentrations in the tissues of most marine organisms. The flux of DFAAs released by injured animals correlates with the attraction of the mud snail Ilyanassa obsoleta to the injured animals (Zimmer et al. 1999). The DFAAs are not leaking out from intact prey at concentrations detectable from a distance, but rather they are taken up from water and retained in the body of the prey (Derby and Zimmer 2012). For example, phyllosoma larvae of the Japanese lobster Panulirus japonicus (Souza et al. 2010) and of the hard clam Mercenaria mercenaria (Zimmer et al. 1999) absorb DFAAs from environmental water. Thus, DFAAs that stimulate food searching are not leaking out from the body surface of living intact organisms.
Excretion is another way that chemicals are released into the environment. The urine of the helmet crab Telmessus cheiragonus contains common amino acids and nitrogenous compounds, with taurine, urea, and ammonia being the most abundant compounds, ranging in concentration from 49 to 248 μM, while the other compounds are present at < 20 μM (Kamio et al. 2005). Crustaceans and fish use urine to communicate with conspecifics in such social contexts as courtship (Kamio et al. 2000, 2014; Yambe et al. 2006a), fighting (Oyama et al. 2020), and aggregation (Horner et al. 2006). In the search for their food, omnivorous consumers detect common molecules, including DFAAs, and specialist consumers detect specific molecules (Kamio and Derby 2017). The metabolites in the excreted urine reflect the feeding history of the organisms, and prey can avoid predators through the use of chemical cues in their urine (Poulin et al. 2018). Gas exchange through the gills and/or the body surface releases carbon dioxide that can also attract predators (Caprio et al. 2014). Carbon dioxide is always leaking out of the body of organisms and predators and parasites can track it as a cue.
Exocrine glands release chemical compounds into the environment. The sea hare Aplysia californica releases chemical compounds from its ink and opaline glands (Johnson et al. 2006), and some of these compounds are cues that evoke alarm responses in conspecifics (Kicklighter et al. 2007, 2011). A. californica also releases deterrents (Kamio et al. 2010a, 2010b) and molecules that confuse predators (Kicklighter et al. 2005); these defensive molecules are only released when the organism is in danger.
Leakage of chemical cues, as opposed to controlled release, occurs in some fish. Unconjugated steroids that stimulate male courtship behavior appear to be released by females in a nonspecialized way (i.e., leaked) across the gills (Scott and Ellis 2007).
To summarize, some chemical cues are released from organisms intentionally to deter predators, attract mates, and alarm conspecifics, and others are released accidentally or unintentionally in the case of injury, excretion, and respiration.
Chemosensory systems in aquatic animals
All organisms sense chemical cues, including bacteria (Ortega et al. 2017), algae (Kinoshita et al. 2017), yeast (Yashiroda and Yoshida 2019), plants (Kong et al. 2018), invertebrates, including poriferans (sponges), ctenophores, placozoans, cnidarians, polychaetes, gastropods, cephalopods, crustaceans, and echinoderms (Derby 2020), and vertebrates, such as fish (Laberge and Hara 2001), amphibians (Kikuyama et al. 2013), reptiles, birds, and mammals (Wyatt 2014). Thus, aquatic animals can detect and adaptively respond to the chemical cues in water, with the exceptions of deep diving marine mammals such as elephant seals and whales in which olfaction is reduced (Bird et al. 2020; Kishida 2021).
Aquatic organisms smell and taste cues in a different way than do humans. Terrestrial mammals smell volatile compounds that come into their nose, and they taste compounds that contact the tongue. The ability of the olfactory sense to detect volatiles makes olfaction a distance chemoreception and gustation, by definition, a contact chemoreception. Thus, the difference between olfaction and gustation is relatively clear in terrestrial mammals. Some terrestrial mammals, though not adult humans, have a vomeronasal organ (VNO) that detects volatile and nonvolatile pheromones (Keverne 1999).
In the aquatic environment, the definition of smell and taste cannot be the same as in the terrestrial environment because unlike terrestrial animals, aquatic organisms and their chemical senses are bathed entirely in water (Fig. 1). Thus, mobile molecules (distant cues) in water are mainly water soluble. However, low water-soluble amphiphilic compounds can become mobile by forming micelles, and lipids can become mobile when they are carried by lipoproteins (Hevonoja et al. 2000). Immobile molecules (contact cues) can be either water-insoluble and soluble molecules.
Chemosensory systems of aquatic animals differ from those of terrestrial mammals. For example, the Caribbean spiny lobster Panulirus argus has aesthetasc sensilla on their antennules (Derby 2021) that detect cues for social communication. Cues of food are detected by sensilla called distributed chemosensilla located on all body parts, including the mouthparts (Kozma et al. 2020b). Fish, which are vertebrates and thus phylogenetically close to mammals, have olfactory organs in their nostrils and taste cells in their mouth, but the common carp Cyprinus carpio has taste buds on the outside of its mouth and the channel catfish Ictalurus punctatus has taste buds over its entire body (Atema 1971) and is described as a “swimming tongue” (Caprio et al. 1993). The chemosensory organs of lobster and fishes and other marine animals differ both phylogenetically and morphologically; however, functionally, they are similar in terms of detecting the same set of chemical cues in the aquatic environment.
The detection of chemical cues starts with the binding of the cue molecules to chemoreceptor proteins on the chemosensory organs. Chemoreceptor proteins in vertebrates, including fish, are G Protein-Coupled Receptors (GPCRs). Chemosensory GPCRs include trace amine-associated receptors (TAARs), vomeronasal receptor type 1 and 2 (V1R, V2R), rormyl-peptide receptors (FPRs), and taste receptor type 1 and 2 (T1R, T2R). Vertebrates also have a different class of important—though numerically less abundant—chemoreceptor proteins, namel, ionotropic receptors, which include transient receptor potential (TRP) channels, epithelial sodium channels (ENaCs), and MS4A receptors (Greer et al. 2016). In crustaceans, chemoreceptor proteins are mostly ionotropic receptors (IRs), TRP channels, and gustatory receptor-like receptors (GRLs). For example, the Caribbean spiny lobster P. argus expresses hundreds of IRs in its chemosensory organs and has even more IRs expressed in the olfactory organ, which is the lateral flagellum of antennule, than in dactyls, which has distributed chemosensilla (Kozma et al. 2020a). Single-cell transcriptome analysis of olfactory sensory neurons of P. argus revealed that individual cells express relatively few IRs, the identity of which differs across cells (Kozma et al. 2020b). Chemoreceptor genes and the proteins that they encode have been identified in other marine invertebrates, including sea hares, octopus, sea urchins, and starfish (reviewed in Derby et al. 2016a). Rapid progress is being made in this field, so detailed results on their distribution and functions should be forthcoming.
Sex pheromones
The first pheromone molecule to be identified was in the silk moth Bombyx mori (Butenandt et al. 1961), and the first pheromone identified in aquatic vertebrates was in fish (Colombo et al. 1980). Since then, other pheromone molecules have been identified, including those in invertebrates, such as polychaetae worms (Zeeck et al. 1988) and mollusks (Painter et al. 1998). In this article, we focus on two major groups of animals that are relatively important in fisheries and aquaculture: decapod crustaceans and fish.
Sex pheromones in decapod crustaceans
The chemosensory biology of invertebrates is best understood in the Crustacea. Sex pheromones in crustaceans have been recognized for decades, and several molecules have been reported as pheromones or candidate pheromones. Chemical communication using pheromones was first reported in premolt female urine of the three-spot swimming crab Portunus sanguinolentus (Ryan 1966). Since then, behaviors induced by pheromones, chemosensory organs that detect pheromones, and the chemistry of pheromones have been studied in multiple crustacean species (Thiel and Breithaupt 2010). The identification of pheromones in crustaceans is challenging, but successful examples include the larval settlement factor of barnacles, which is an α2-macroglobulin-like glycoprotein complex called the “settlement inducing factor” (Matsumura et al. 1998; Pagett et al. 2012). The pheromone used by the male copepod Tigiriopus japonicus is also partially identified as a α2-macroglobulin-like glycoprotein (Ting and Snell 2003). These examples of the successful identification of phermones occurred in small animals which can be reared in aquaria in a laboratory. Thus, success in the culturing of animals may be the key for success in identifying pheromones because the process requires many repeats of behavioral bioassays and sufficient amounts of the target molecules, which in turn necessitates a large number of animals.
Soft female mating (SFM) crabs, which mate just after the female molts and her exoskeleton is soft (Hartnoll 1969), have been model animals for studying pheromones because of their prolonged courtship period (Fig. 2). In SFM species, premolt females release pheromone in their urine and males respond by courtship display and start precopulatory guarding in which a male carries a female. When the female starts molting, the male helps her shed her old exoskeleton and then copulates with her by inserting his gonopods into hers. In the helmet crab T. cheiragonus, which has SFM-type mating behavior (Kamio et al. 2003), females release two functionally different pheromones: a courtship pheromone in the premolt stage and a copulation pheromone in the postmolt stage (Kamio et al. 2002). Maturation of the ovary starts after molting, and females spawn in the absence of males by using sperm stored in the seminal receptacle. The period of pheromone release and male guarding, which is several days long, is an advantage to researchers because it facilitates the collection of competent males and females. Furthermore, the state of reproductive females and the duration before and after pubertal molting can be estimated in the helmet crab by observing the color change of the abdomen to telson and by submissive behavior (Kamio et al. 2021a); in the blue crab Callinectes sapidus, morphological change of the swimming paddle is an additional change (Jivoff et al. 2007; Smith and Chang 2007). These characteristics make SFM crabs, especially the helmet crab and blue crab, suitable materials for studies on chemical communication in mating using sex pheromones.
Female courtship pheromones in decapods
Males of SFM species can distinguish the molting stages of females, i.e., intermolt, premolt, and postmolt, by detecting her pheromones. Premolt females release pheromone that facilitates courtship. Ryan (1966) reported that the female three-spot swimming crab P. sanguinolentus releases a pheromone that induces searching behavior in males with display, such as walking on tips of its dactyls with its body elevated at maximum height above the substratum and extended chelae. Since then, researchers have used searching and courtship behavior as criteria to detect pheromones. Courtship includes all interactions between males and females prior to copulation. In early studies on crab pheromones, two behaviors were used as criteria for identifying the responses of males to female pheromones: searching behavior with a characteristic posture, which is walking on the tips of dactyls with the body elevated to a maximum height and extended chelae (Ryan 1966; Kittredge et al. 1971), and approaching other crabs (Ryan 1966; Kittredge et al. 1971). However, some components of these behaviors, including grasping other crabs, standing high on legs, extending chelae, and epipod waving, can be observed in males that are fighting or searching for food rather than courting females (Kamio et al. 2014). Consequently, more reliable and specific criteria for identifying male courtship have been used; one of which is courtship stationary paddling, a display that includes the paddling of swimming legs, which is characteristic to the blue crab C. sapidus (Fig. 3) (Teytaud 1971; Gleeson 1980; Kamio et al. 2008, 2014). Despite its high specificity to mating behavior, this display is context dependent, and males tend to skip the display (Kamio et al. 2008); thus, use of this behavior in bioassay-guided fractionation assays tends to yield false negatives. Precopulatory male-guarding behavior of artificial objects, such as sponges, stones, or golf balls, has also been used as a criterion of male sexual responses for the identification of female courtship pheromone molecules in the hair crab Erimacrus isenbeckii (Asai et al. 2000), helmet crab T. cheiragonus (Kamio et al. 2000), and European green crab Carcinus maenas (Hardege et al. 2002) (Fig. 3). In this context, male helmet crabs embrace a urethane sponge containing 20 µL of female urine, and then palpate the sponge as they do to real premolt females without biting the sponge (Kamio et al. 2000). Using these sponges in assays of precopulatory guarding has advantages: it can help identify pheromones without requiring interactions among animals, and it can help distinguish courtship behavior from feeding behavior due to the absence of biting on the sponge. Thus, various aspects of courtship behavior have been used to evaluate the responses of males to pheromones depending on the nature of each species. For example, courtship stationary paddling of C. sapidus is very specific to courtship behavior but not an efficient characteristic in bioassays, while guarding artificial objects, as used in studies on the hair crab, helmet crab, and European green crab, is specific and more efficient. The sponge assay was originally believed to be the ideal bioassay for all SFM species; however, because blue crab males are aggressive to sponges spotted with premolt female urine, the sponge assay is of limited use in this species (Kamio, unpublished data). Thus, courtship pheromones in urine released by premolt females is common in species; however, the behavioral character of males in the bioassay differs from species to species even within SFM species. Thus, the design of the behavioral bioassay for each species needs to be specific for that species and based on a deep understanding of the species’ mating behavior from searching and courtship, to copulation and agonistic behaviors.

Sources: a–c Kamio et al. (2000); modified with permission of the Zoological Society of Japan. d Hardege et al. (2002); reproduced with permission of Inter-Research Science Center. e Photograph courtesy of CM Rowell
Courtship behaviors used in bioassays. a–c Sequential images of a male helmet crab Telmessus cheiragonus grasping a urethane sponge. d Male European green crab Carcinus maenas guarding a stone. e Male blue crab Callinectes sapidus showing courtship stationary paddling.
Urine contains the courtship pheromone. Urine collected from the nephropores (i.e., the opening of the antennal gland or excretory pore) is a clean material that is suitable for chemical analysis of the pheromone because it does not contain molecules and inorganic salts from other body parts or seawater. Ryan (1966) indicated that the courtship pheromone is released in the premolt female urine by showing that capping the nephropore of females decreases the responses of males (Ryan 1966). Gleeson (1980) confirmed the pheromonal activity of urine collected from nephropores, and such urine has been used to study the chemical nature of the pheromone in the blue crab (Gleeson et al. 1984; Kamio et al. 2014), European green crab (Bamber and Naylor 1997; Hardege et al. 2002, 2011), and helmet crab (Kamio et al. 2002, 2005; Yano et al. 2016). Postmolt females release more urine than premolt females (Yano et al. 2016), and males respond to the urine by showing courtship behavior (Kamio et al. 2002). Urine collection from tiny crustacean species, such as copepods, amphipods, and isopods, is not possible, although the females of these species do have pheromones (Thiel and Breithaupt 2010). Urine collection methods are established in these crabs and some lobsters and urine is thus available for biological and chemical studies.
The chemical nature of courtship pheromones has been reported for several species. The pheromone of the striped shore crab Pachygrapsus crassipes released in seawater is a heat-stable, low-polar, non-ionic lipid, and the molting hormone, crustecdysone, which possesses these physicochemical characteristics, has been reported to elicit the courtship response in males of the striped shore crab, the Pacific rock crab Romaleon antennarium (formerly Cancer antennarius), and the yellow rock crab Metacarcinus anthonyi (formerly Cancer anthonyi) (Kittredge et al. 1971). However, these results have not been replicated in these species, and crustecdysone (Fig. 4) does not induce courtship behavior in the blue crab (Gleeson et al. 1984) or European green crab (Gleeson et al. 1984; Hardege et al. 2002). Crustecdysone may be a part of the sex pheromone mixture, but further research is needed by combining it in tests of other sex pheromone candidate molecules.
In contrast to the lipophilic character of the pheromone of the the striped shore crab, pheromones reported in other well-studied crabs are highly polar. The pheromone present in the urine of blue crab was reported to be highly polar small molecule(s) (< 1000 Da; Gleeson 1991; Kamio et al. 2014). NMR-based metabolite analysis (Kamio 2009) of premolt female urine revealed a biologically novel compound, N-acetyl-D-glucosamino-1,5-lactone (NAGL) (Fig. 4), a potential indicator of premolt and molting animals (Kamio et al. 2014). NAGL alone does not elicit courtship behavior, but because of its physicochemical properties, it is a premolt indicator and a candidate member of the pheromone blend. NAGL was also found in the urine of helmet crab as a premolt indicator whose concentration in the urine increases towards molting and is released at the highest amounts immediately after molting because urine release increases at that stage (Yano et al. 2016). NAGL is present in the urine as a tautomer mixture dominated by an acid, 2-acetamido-2-deoxygluconic acid (Fig. 4) (Kamio et al. 2017). The chemical character, high polar, and small size of this molecule is the same as that of the pheromone of this species and, therefore, it is a candidate pheromone molecule which works together with other molecules.
The pheromone in the urine of the European green crab is a highly polar small compound (< 1000 Da) (Hardege et al. 2002) and is reported to be uridine diphosphate (UDP) (Hardege et al. 2011). In the hair crab, a ceramide mixture (Fig. 4) isolated from its environmental water was identified as a candidate pheromone (Asai et al. 2000). Although these ceramides have been synthesized (Asai et al. 2001), they have not yet been tested for pheromonal activity in bioassays on male hair crabs. Further research to develop a suitable stable behavioral bioassay is needed in hair crabs.
Lipophilic cuticular hydrocarbons have been reported to be contact sex pheromones in the hermaphroditic marine shrimp Lysmata boggessi based on behavioral bioassays using as response criteria the grasping and touching of pheromone-treated plastic tubes (Zhang et al. 2011). Newly molted euhermaphrodite-phase shrimp contain a bouquet of odor compounds. Of these, (Z)-9-octadecenamide is the key odor compound with hexadecanamide and methyl linoleate enhancing the bioactivity of the pheromone blend. A water-soluble compound, uridine triphosphate (UTP), was reported as a candidate component of the soluble sex pheromone bouquet in the marine shrimp Lysmata wurdemanni, using as criteria courtship behaviors, approach, and following (Zhang et al. 2020).
Some of the molecules reported to date as the pheromone or candidate pheromone are related to the biosynthesis and degradation of chitin that occurs in molting. NAGL is an oxidized form of N-acetylglucosamine; UDP is produced during chitin synthesis; and crustecdysone is produced in the Y-organ and released in the hemolymph (Chang and Mykles 2011). These molecules could evolve into pheromones when exposed to cues that are markers of molting; however, these molecules do not carry information that indicates sex since they are produced and released in both males and females. Thus, there should be molecules that indicate “female” in the urine. Ceramides and hydrocarbons are not metabolites or hormones that are related to molting.
Male courtship pheromone: bidirectional chemical communication in decapods
Males, not only females, of SFM species release pheromones, thus establishing a bidirectional chemical communication system during courtship. Blue crab C. sapidus premolt females are attracted to males in Y-maze experiments (Gleeson 1991). A component of the courtship display of the males, namely, “courtship stationary paddling,” is an adaptation to their habitat where premolt females are hiding in refuge (e.g., a patch of Spartina grass). In courtship stationary paddling, males produce a forward-directed water current by paddling their swimming legs to deliver their pheromone to females that are in hiding or behind a barrier (Kamio et al. 2008).
Females evaluate the quality of males in addition to finding them. In the anomuran crab Hapalogaster dentata, postmolt females choose males that have more sperm by sensing water-borne pheromones released from males (Sato and Goshima 2007). In environments where the water is turbid or dark and thus where visual communication is not effective, it is believed that organisms use chemical communication and bidirectional communication to find and recognize each other. The crustacean species discussed in this paper are generally active at night and living in turbid water where they cannot rely on visual cues; thus, they rely on chemical cues. Urine collection from the nephropore in anomuran species has not yet been established. The location and morphology of the nephropore should be studied to collect urine for use in studies on chemical communication in this group.
Female copulation pheromone in decapods
A two-step chemical communication system involving both courtship pheromones and copulation pheromones has been identified during mating of the helmet crab T. cheiragonus (Kamio et al. 2002) (Fig. 2). In that study, the sponge assay in the helmet crab clearly distinguished the courtship pheromone from the copulation pheromone (Fig. 5), with males showing precopulatory guarding of sponges containing premolt female-conditioned water and copulation behavior with sponges containing postmolt female-conditioned water. Moreover, males deposited sperm plug on the sponge. The copulation pheromone is a small molecule(s) of < 1000 Da and has not been identified yet.

Source: Kamio et al. 2002; reproduced with permission of Inter-Research Science Center
Evidence of copulation pheromone in a male helmet crab Telmessus cheiragonus. a Male crab guarding a urethane sponge containing premolt female tank water. b Male crab copulating with a urethane sponge containing postmolt female tank water. c Sperm plug deposited on gonopore of postmolt female. d Sperm plug deposited on sponge.
Studies on other crustacean species have focused on pheromones that elicit courtship behavior in males or did not distinguish copulation from courtship. All SFM species may have the copulation pheromone because the males of these species can distinguish postmolt females from premolt females.
Olfactory organs for pheromone detection in decapods
Aesthetascs on the outer flagellum of antennules are the pheromone detector organs in SFM crabs (Gleeson 1980; Kamio et al. 2005). Integrated responses detected in electrophysiological recordings from chemosensory neurons in the outer flagellum of the helmet crab T. cheiragonus show that female urine produces larger responses than does male urine (Kamio et al. 2005). In the blue crab C. sapidus, olfactory sensory neurons in the outer flagellum are sensitive to NAGL, as indicated by calcium-imaging studies (Kamio et al. 2014). Chemosensory neurons specifically responsive to premolt urine have not been reported. Electroantennogram (EAG)-coupled chromatography in studies of insect pheromones has shown responses from pheromone receptor neurons (Batista-Pereira et al. 2006), and electrophysiological recordings in crustaceans are expected to show similar responses from pheromone receptor neurons. However, the antennules of crustaceans are more complex than those of insects, with an outer flagellum of the blue crab bearing 650–700 aesthetasc sensilla and each sensillum innervated by between 40 and 160 olfactory sensory neurons (Gleeson et al. 1996). Electrophysiological and calcium imaging methods are useful for studying the response properties of single neurons but not for guiding the purification of pheromone molecules. EAG measurements in crustaceans can be utilized to evaluate the ability of their chemical senses in an ecological context. For example, EAG studies using the terrestrial coconut crab Birgus latro and the land hermit crab Coenobita clypeatus identified volatile compounds detected by the antennules of these crabs, representative of the evolutionary transition of crabs from sea to land (Stensmyr et al. 2005; Krång et al. 2012). These EAG studies on terrestrial crabs used volatile molecules delivered in air. Techniques have been developed to record EAGs from the marine shrimp Palaemon elegans (Machon et al. 2016), and these techniques have been used on the hydrothermal shrimp Mirocaris fortunata to show their ability to locate hydrothermal vents by tracking water-borne sulfide (Machon et al. 2018). The application of electrophysiological recording from chemosensory organs for the screening of pheromone molecules has not yet been successful; however, the method used in the studies of P. elegans and M. fortunata has potential to be used for selecting chemical cues detected by antennules.
Decapod pheromone glands
Where the pheromones are produced and stored is not known, but there are candidate organs that produce the pheromones. Green glands (antennal gland) are such a candidate organ because they produce urine (Cameron and Batterton 1978); however, these glands have not yet been reported as containing pheromone molecules. Another possible source of the urine pheromone is two clusters of nephropore rosette glands in the American lobster Homarus americanus that lie along the ureter, with one opening into the bladder and the other opening to the exterior (Bushmann and Atema 1996). These two duct systems could allow these glands to release their products into the environment with or without urine release (Bushmann and Atema 1996). In addition to the pheromones being released into the urine, other release sites for pheromones are likely. The postmolt urine of the helmet crab T. cheiragonus does not contain the copulation pheromone, although the release site of this pheromone has not been identified (Kamio et al. 2002). In the freshwater prawn Palaemon paucidens, sternal glands under the sternal plate of females that open at the sternal tegument could be a release site of pheromones. The contents of the sternal gland disappear just after molting when the female mates (Kamiguchi 1972), indicating that the contents were released to the environment. Other candidate pheromonal glands include the cement gland, which attaches eggs to the abdominal region, and the tegumental glands, which occur over the body of crustaceans (Talbot and Demers 1993) and which secrete molecules, including enzymes, into the environment (Stepanyan et al. 2005; Schmidt et al. 2006). Some tegumental glands could be the release site of the copulation pheromone of the helmet crab.
Application of decapod pheromones in fisheries and aquaculture science
Knowledge of chemical communication could be used in the aquaculture of crustacean species by facilitating the management of brood stock, hatching, growth, and harvesting (Barki et al. 2010). Sex pheromones could be used to collect sexually active animals in the field or in aquaculture facilities to synchronize and asynchronize mating activity. Cues that accelerate (conspecific, heterospecific prey) or delay (predator) settlement could be used to synchronize metamorphosis. Some compounds involved in water-borne dominance cues could be removed by filtering through charcoal filter or destroyed by UV irradiation, resulting in decreased suppression of growth. Incorporation of pheromone-based attractants in feed could stimulate food intake and decrease feed waste. In shrimp in which sex determination depends on population structure (Chiba et al. 2013), conspecific chemical cues might control the sex of growing animals to fit market demands. Chemical cues from live animals can and are being used in certain fisheries. For example, in the fishery for the blue crab C. sapidus, males are used to attract and catch premolt females for the soft-shell crab industry (Hungria et al. 2017), and premolt females can be used to catch male crabs. However, crustaceans used as attractants in this way are stressed and often die in the traps (Hunt et al. 1986). Thus, identification, artificial synthesis, and the use of synthetic pheromonal molecules rather than the live animals themselves could improve the efficiency and efficacy of such fisheries. Male pheromones might be used as an artificial bait to attract premolt females. For example, laboratory experiments show that female blue crabs are preferentially attracted to chemical cues released by males, although the attractant molecules released from the male blue crabs has not been identified yet (Gleeson 1991). Thus, there is a demand for the application of pheromones in fisheries and aquaculture of crustacean species. Currently, some fisheries are applying chemical communication cues of crustacean species using live animals as a resource of pheromone. The development of synthetic pheromone could replace the live baits.
The sex pheromone in crustaceans has been a focus of research in biology and fisheries but it is not yet fully understood. UDP and hydrocarbons have been reported as pheromone in a limited number of species, but the pheromonal activity of these molecules has to be tested in related species and the concentration or amount of these molecules has to be studied in the urine and cuticle of other species. Pheromonal activity and concentration of the pheromone candidate molecule in the urine, NAGL and ceramides should be studied extensively in crustacean species. This research combined with the search of new candidate molecules will lead to a better understanding of pheromone because it might be a mixture of compounds.
Fish sex pheromones
Chemical communication in fish had been taken into consideration in the design of animal rearing systems since at least the 1960s. In connected pond systems where water flows from an upper pond to a lower pond, animals in the lower ponds are exposed to pheromones released from animals in the upper ponds. At the Nikko field station (National Research and Development Agency, Japan Fisheries Research and Education Agency), lacustrine sockeye salmon Oncorhynchus nerka nerka males are held in an upper pond to avoid exposure to female pheromones that induce over-maturation of males, and females are held in a lower pond to accelerate ovulation induced by male pheromones. In the field, maturation in males of this fish species generally progresses earlier than that of females; therefore this setting results in mature males and females at the same time in the laboratory. Detailed investigations of sex pheromones have been performed on the goldfish Carassius auratus since the 1980s. Hormones that endogenously promote ovarian maturation are metabolized in the female goldfish and released as sex pheromones via their urine or gills. Thus, we recognize “hormonal pheromones” as representative sex pheromones in fish. Several hormonal molecules function as pheromones that elicit sexual behavior (releaser effect) or physiological changes (priming or primer effect) in many species. The olfactory organs of fish respond only to a limited number of chemical species dissolved in water, such as amino acids, steroids, bile acids, and prostaglandins (Hara 1994), and molecules identified as pheromones in fish belong to these categories. Chemical communication in each fish species has been shaped through the evolution of their reproductive behaviors. The sex which makes the nest for spawning or the sex which determines the spawning area has evolved to secrete the sex pheromones. Here, we review pheromones in female and male fish which have been chemically identified, and we also suggest possible applications of sex pheromones in the fisheries and aquaculture of fish.
Female sex pheromones in fish
Identified molecules, route or source, and effects of sex pheromones released by female fish are summarized in Table 1. Hormonal pheromones in Cypriniformes are among the most well-understood fish pheromones. We illustrate here essential points in sex pheromones of the goldfish, as an example of sex phermones in the cyprinids (Fig. 6), because many excellent reviews have been already published (Kobayashi et al. 2002; Stacey and Sorensen 2006; Sorensen and Wisenden 2015). Firstly, it has been suggested that the male-attracting pheromone is already released by the female at the vitellogenic stage in which there is a high level of plasma 17β-estradiol (E2) (Kobayashi et al. 2002). The presence of this pheromone was demonstrated in experiments (Yamazaki and Watanabe 1979; Yamazaki 1990) as follows: males or females that were treated with E2 after hypophysectomy or gonadectomy were recognized and chased by males or by females that were treated with 17α-methyltestosterone (synthetic androgen) after hypophysectomy or gonadectomy, but these test fish used do not reach the spawning stage. The chemical cue released from E2-treated females may be a metabolite of E2 or an unknown chemical that is not biosynthetically derived from E2 but secreted by the hormonal action of E2. When the ovary sufficiently completes vitellogenesis, the surge of luteinizing hormone (LH) occurs, induced by environmental cues, such as an increase in water temperature, rainfall, water replacement of the aquarium, change in water flow speed, and/or the addition of spawning substrate to the water. Thereby, the maturation-inducing steroid (17α,20β-dihydroxy-4-pregnen-3-one [17,20β-P]) is secreted from the follicles in the ovary, which induces oocyte maturation. At that time, free 17,20β-P and its sulfate are released into the water from the female’s gills, kidney, and intestine. These steroids exert the priming effects of sex pheromones on reproductively mature males, inducing an LH surge from the male’s pituitary, a secretion of 17,20β-P in the testis due to the LH, and then elevation of the milt volume (Dulka et al. 1987; Stacey et al. 1989). 17,20β-P also increases male fertility, such as by increasing sperm motility, male competitive spawning, and the number of ejaculated sperm (Zheng et al. 1997; Hoysak and Stacey 2008). Male primer responses to 17,20β-P have also been confirmed under natural conditions (Olsén et al. 2006). 17,20β-P and 17,20β-P-sulfate, which are released at the preovulation stage, activate male searching behavior (Sorensen et al. 1989; Defraipont and Sorensen 1993; Poling et al. 2001). Androstenedione, which is released from pre-ovulatory females and mature males, induces aggressive behavior of mature males, suggesting that mature males keep away conspecific individuals not targeted for spawning behavior (Poling et al. 2001; Sorensen et al. 2005b). Curiously, an avoidance reaction of mature male goldfish to 17,20β-P has also been observed using Y-maze tests (Bjerselius et al. 1995). After ovulation, prostaglandin F2α (PGF2α), whose release is correlated with the rupture of ovarian follicles and the presence of ovulated eggs in the ovarian lumen (Stacey and Liley 1974), not only internally stimulates the female brain and induces female sexual behavior (Stacey and Peter 1979) but also externally induces male courtship (chasing) and male spawning (ejaculation) behaviors (Sorensen et al. 1988) when PGF2α and 15-keto-PGF2α are released with female urine into the ambient water (Appelt and Sorensen 2007; Sorensen et al. 2018). Urinary pulses of a sexually receptive female increase according to mature male activity (Appelt and Sorensen 2007). Furthermore, F-series prostaglandins (PGFs) exert slightly priming effects on mature males (Sorensen et al. 1989). In electro-olfactogram (EOG) recordings of the olfactory epithelium response to the hormonal pheromones, the response to 17,20β-P did not differ either between the sexes or among stages of sexual maturity. The olfactory epithelia of both sexes are able to detect PGF2α and 15-keto-PGF2α, but mature males are more sensitive to the PGFs. The thresholds of olfactory responses to 17,20β-P and 15-keto-PGF2α are approximately 10–12 M in mature males. In many species, the olfactory and behavioral responses to sex pheromones depend on androgens (Yamazaki 1976; Yamazaki and Watanabe 1979; Cardwell et al. 1995; Stacey and Kobayashi 1996; Yambe and Yamazaki 2000; Murphy and Stacey 2002; Belanger et al. 2010). In the neuroendocrine system, 17,20β-P and PGFs appear to act through distinct pathways (Chung-Davidson et al. 2008; Lado et al. 2013). Owing to common hormonal substances in vertebrates, the species specificity of sex pheromones in cyprinids has been obscure for a long time. In the common carp Cyprinus carpio and goldfish, however, species-specific substances that are released from immature or mature females and have conspecific attracting effects seem to be polar molecules that are as yet unidentified (Levesque et al. 2011; Lim and Sorensen 2011). These unidentified chemicals likely act synergistically to PGFs and may help reproductive isolation in cyprinids.

Modified from Yambe (2013), with permission of The Japanese Society of Fisheries Science
Schematic representation of of sex pheromones in the goldfish Carassius auratus. 17,20β-P 17α,20β-Dihydroxy-4-pregnen-3-one, AD androstenedione, E2 17β-estradiol, KT 11-ketotestosterone, LH luteinizing hormone, PGFs prostaglandins F-series. Text in italics indicates pheromonal chemicals and their effects in males. Dotted arrows indicate internal hormonal movements.
There are other hormonal pheromones categorized as female pheromones in cyprinids. The sex pheromones 17,20β-P and PGFs in cyprinids, including common carp, have been found to have effects similar to those in goldfish (Stacey et al. 1994; Cardwell et al. 1995; Lim and Sorensen 2011). The gynogenetic crucian carp Carassius auratus langsdorfii is an all-female species. Under experimental conditions, spawned eggs of the crucian carp begin to develop when stimulated by sperm of other species (Dong et al. 1997). In the field, ovulatory females possibly attract mature males of other species by secreting PGFs and utilizing their sperm to stimulate the embryogeny of their own eggs. A releaser pheromone of the loach Misgurnus anguillicaudatus, 13,14-dihydro-15-keto-PGF2α, is a secondary metabolite of PGF2α (Kitamura et al. 1994b; Ogata et al. 1994) and induces male courtship behavior in an ovulating female, with a threshold of 10–13 M based on EOGs. In the channel catfish Ictalurus punctatus and blue catfish I. furcatus, results from the injection of PGF2α suggested that a male-attracting pheromone was the PGF2α secreted from females (Broach and Phelps 2011). The zebrafish Danio rerio has been well studied in terms of its olfactory system and chemosensory neuroethology (Yoshihara 2014). The olfactory system of zebrafish detects PGFs and 17,20β-P-sulfate (Friedrich and Korsching 1998; Belanger et al. 2010). In another study, PGF2α was also identified as a sex pheromone that induces male sexual behavior, and its receptor and the neural pathway were also shown (Yabuki et al. 2016). E2 from the mature female guppy Poecilia reticulata seems to attract mature males (Johansen 1985). PGFs and steroids have been assumed to be the common pheromones in teleost fish for a long time, based on the reports of the olfactory response to PGFs in several species, including cyprinids (Cardwell et al. 1991; Kitamura et al. 1994a).
Salmonid fishes are commercially important worldwide. Conspecific chemical cues in Salmo species have been investigated since the 1970s because of biological interests and application in fisheries. In the Atlantic salmon Salmo salar, releaser sex pheromones that attract mature males are present in ovulated female urine (OFU) (Olsén et al. 2002). This pheromone has not yet been identified chemically. OFU, PGFs in the urine, and coelomic fluid of ovulated females were found to have priming effects that elevate plasma sex steroids in males (Moore and Waring 1996; Olsén et al. 2001). However, in estimating the concentrations of PGFs, unknown chemicals will also be present in the pheromonal solutions. PGFs that induce releaser (Laberge and Hara 2003) and priming effects (Moore et al. 2002) of the male brown trout Salmo trutta are prospective candidates of sex pheromones in the coelomic fluid of ovulated females. Taking the olfactory thresholds and the secreted concentrations into account, we expect the presence of unidentified pheromonal compounds. Although the contribution of PGFs is not yet known, sperm from primed males exposed to a mixture of urine and coelomic fluid of ovulated females generate more offspring in brown trout (Hellstrom et al. 2013).
Not all pheromones secreted by females are hormonal molecules or their derivatives. Genital cavity fluid in mature females and males of the pond smelt Hypomesus olidus contains sex pheromones that attract mature males and induce male courtship behavior. The active substances are likely peptides or proteins (Okada et al. 1978). Proteinogenic amino acids are candidate female sex pheromones in the rose bitterling Rhodeus ocellatus ocellatus (Kawabata 1993). A nuptial-colored male of rose bitterling first pecks the abdomen of an ovulatory female; then the female uses an extended ovipositor to inject ovulated eggs into the exhalent of a bivalve, which is the spawning substrate for females. The male then ejaculates near the inhalant siphon of the bivalve, and the sperm is taken into the siphon and fertilizes the female’s eggs that are present on the gill filaments of the bivalve. In the study of Kawabata 1993, 20 amino acids were studied, of which five, l-cysteine, l-serine, l-alanine, l-glycine, and l-lysine, induced pecking and ejaculation at the highest activity. The author hypothesized that these five amino acids induce the pecking behavior of males to guide females to bivalves during the night and induce ejaculation during the day. However, the concentrations of the amino acids in ovarian fluid and the exhalent water were not measured, and the thresholds of behavioral responses were not determined in this study.
The sex pheromone of the puffer fish Takifugu niphobles is unique because it is well-known as one of the most potent marine toxins, tetrodotoxin (TTX) (Matsumura 1995). In the breeding season, mature males and females gather on the beach to spawn. TTX accumulates in the oocytes and then transferred to the ovarian cavity fluid through the chorion during ovulation. TTX acts as a male-attracting pheromone by leaking from the ovarian cavity into the environment. The threshold pheromone concentration to elicit a behavioral response was estimated to be about 1.5 × 10–12 M. Interestingly, immature juveniles of Takifugu rubripes are also attracted to TTX (Okita et al. 2013), suggesting that the behavioral responses of puffer fish to TTX may be not sexually specific.
In the rainbow trout Oncorhynchus mykiss, releaser sex pheromones are present in ovulated female urine (Yambe and Yamazaki 2001b). Urine or bile of the ovulated females exerts priming effects that increase the levels of plasma steroid hormones and expressible milt in males (Scott et al. 1994; Vermeirssen et al. 1997; Vermeirssen and Scott 2001). Evidence from electrophysiological studies suggest that bile acids are candidate sex pheromones in rainbow trout (Giaquinto and Hara 2008). The sex pheromones of rainbow trout have not yet been identified chemically, suggesting a possibility of unidentified pheromones other than hormonal pheromones (Liley and Rouger 1990; Scott et al. 1994; Sato and Suzuki 2001; Laberge and Hara 2003).
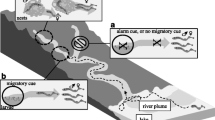
The masu salmon Oncorhynchus masou masou is a convenient species to study sex pheromones in salmonid fishes because they have two life-history forms (Fig. 7). Anadromous females (body length: 40–60 cm) are suitable for urine collection, and river resident males (body length: 20 cm) are easy to handle as test fish in odor experiments. The OFU contains a male-attracting pheromone (Yambe et al. 1999), whereas the urine of spermiating males of anadromous forms repel immature males (Yambe et al. 2006b). Regardless of sex, mature masu salmon have been shown to have a higher kidney somatic index and urine flow rate than immature fish (Yambe and Yamazaki 2006). The function of the kidney or urinary bladder may be enhanced in individuals emitting chemical cues (see below for tilapia). The elution profile from gel chromatography showed that retention times of some hormonal pheromones were not coincident with a peak of active fractions from OFU that attracted male masu salmon. Bioassay-guided fractionation revealed an active compound that was identical to l-kynurenine in spectral and chromatographic properties (Yambe et al. 2006a). l-Kynurenine is one of the amino acids metabolized from l-tryptophan in vertebrates, and it elicits a spermiating male-specific behavior at even picomolar concentrations in masu salmon. In preliminary Y-maze tests, spermiating males were attracted to l-kynurenine. The olfactory epithelium of spermiating males was electrophysiologically more sensitive to l-kynurenine than that of sexually inactive males (immature males and spent males) and ovulated females. The electrophysiological threshold was 10–14 M in spermiating males, whereas d-kynurenine elicited a negligible response in electrophysiological and behavioral tests. Namely, mature male masu salmon recognize the different chiral forms. HPLC showed that l-kynurenine in OFU was 10–4 to 10–5 M and was higher than in urine of spermiating males and immature females. l-Kynurenine concentrations in the OFU of rainbow trout and brown trout were 1% of that in masu salmon. Although the level of l-kynurenine in the urine of rainbow trout or brown trout is sufficient for olfactory responses, these species would not encounter such a high level in natural streams, indicating the species specificity of l-kynurenine, as suggested by Y-maze tests (Yambe and Yamazaki 2001a). Moreover, OFU and 10–9 M l-kynurenine elevated plasma levels of sex steroid hormones in spermiating males (Yambe et al. 2008). Therefore, the tryptophan metabolites in the urine would have not only releaser effects but also priming effects of sex pheromones in masu salmon. To test the effect of l-kynurenine in the field, the pheromone trapping technique is being developed for masu salmon using large-scale attraction tests instead of experimental troughs as used in previous studies (Yambe et al. 1999). Attraction of mature males to l-kynurenine during the spawning season in natural environments was tested using pheromone traps made from a wooden partition, concrete block, and a metal shelf (Fig. 8) (Yambe, 2013). l-Kynurenine at 10–4 M and a control (distilled water) were diluted with river water and pumped into the trap. Many males and a few females were captured at the peak of the spawning season in the traps baited with l-kynurenine. This result indicates that l-kynurenine attracts mature male masu salmon in natural rivers. However, since sex pheromones generally exist as pheromone blends consisting of specific ratios of multiple components, as reported in sea lamprey (Johnson et al. 2014; Brant et al. 2016b; Li et al. 2017), l-Kynurenine and its derivatives should be tested in combination and at different ratios in future studies.
Schematic representation of the life-history and reproductive ecology relating to sex pheromones in the masu salmon Oncorhynchus masou masou. The structural formula of l-kynurenine (Kyn) is given in the urine plume (yellow shading). Adult mature males probably behave like precocious males when they are exposed to Kyn, although only precocious males were used in the experiments

Photograph in a is modified from Yambe (2013), with permission of The Japanese Society of Fisheries Science. The photograph has been flipped horizontally from the original, and labels have been added
A pheromone trap for masu salmon O. masou masou in a stream. a Photograph of a pheromone trap. SS, FP, and BTS were temporary and excluded in the experiments. b Diagram of the pheromone trap (view from above). Two cages made of stainless mesh (each cage: 45 × 35 × 95 cm, W × H × L) were separated by a wooden board and stabilized by concrete blocks. A one-way valve constructed of plastic plates was positioned at each entrance (each entrance: 40 × 25 × 30 cm, W × H × L). Kyn and DW were introduced into each cage using the MP. The position of the Kyn and DW were switched alternately at each trial. Fish could swim upstream by passing through a gap between the trap and the shore. The water level was half the height of the cage. The trap was supported by pegs, ropes, and concrete blocks. BTS Blocks between the trap and shore, DW Distilled water, FP frame pipes, Kyn 10–4 M l-kynurenine, MP micro-peristaltic pump, SS stepping stones,
Anadromous masu salmon females often enter the fasting state at the spawning area, and they spawn just once in their lifetime and then die. l-Tryptophan a glucogenic amino acids, and in masu salmon l-tryptophan may be utilized for gluconeogenesis until spawning time. The hydrophilic property of this amino acid pheromone may be advantageous for masu salmon because mature females need to advertise their mating readiness and location more widely to mature males in mountain streams. Indeed, l-kynurenine as a pheromone was identified in a strain of cultured masu salmon maintained in a public hatchery for > 20 years. Recently, we realized that the bioactivity of l-kynurenine in wild male masu salmon was lower than that in cultured males (unpublished data). For wild males, mixtures of l-kynurenine with other metabolites of l-tryptophan may be more potent than the single molecule l-kynurenine. In cultured fish, the components of pheromones may have been simplified by various artificial pressures.
Female pheromones have been reported in species in which females determine the nesting or spawning sites. Relatively limited components in female pheromones may be sufficient for males that have large number of gametes because males generally mate more than once and with various females. Also, a relatively small number of molecules that represents the number of eggs, i.e., PGFs or maturation-inducing steroids that are related to final maturation, may be sufficient cues for males to evaluate females because males commonly select females that have large numbers of eggs.
Male sex pheromones in fish
Identified molecules, route or source, and effects of sex pheromones released by male fish are summarized in Table 2. In goldfish, an increase of milt volume is induced not only by female pheromones, but also by social stimuli from conspecific males (Stacey et al. 2001; Fraser and Stacey 2002). The chemical cues from males seem to be unknown. Androstenedione from the mature male goldfish increases agonistic behavior among conspecific males (Sorensen et al. 2005b). Studies on pheromones of the sea lamprey Petromyzon marinus have progressed dramatically since around 2000 (Buchinger et al. 2015). Sea lampreys are an important issue in the management of ecosystems and fisheries in the Laurentian Great Lakes of North America. The sea lamprey has a complex life-history comprising larval, juvenile, and adult stages. Larvae habit in streams and are filter feeders. Following the larval stage, they metamorphose into parasitical juveniles and migrate downstream into the Atlantic Ocean or the Great Lakes. Later, the adult sea lamprey migrates into streams to spawn. A spermiating male makes a nest for ovulated females (Buchinger et al. 2015). In itself this species is not itself useful for fisheries, but it is parasitic and causes enormous damage to the lake char Salvelinus namaycush, which is a fishery target in the Great Lakes.
There are two types of sea lamprey pheromones: migratory pheromones, which are released by larvae in rivers and attract adult males and females in lakes to the spawning grounds of rivers, and sex pheromones, which bring together spermiating males and ovulated females (Buchinger et al. 2015). There are more than 40 years of studies on sea lamprey pheromones, involving the isolation of active substances by olfactory or behavioral responses, structural analyses by high-performance liquid chromatography (HPLC), MS, NMR, and confirmation of bioactivity by behavioral experiments. Many of the sea lamprey’s pheromones are bile acids with steroid skeletons and they are species specific.
It was initially suggested that the sex pheromone of this species may be testosterone released from mature males (Teeter 1980). However, based on the concentration of testosterone in male urine and the effective concentration for females, it became apparent that testosterone is only a component of a pheromone mixture. More than 20 years later, a research demonstrated that a sulfate conjugate of bile acid, 3-keto-petromyzonol sulfate (3kPZS), released from the gills of spermiating mature males, was the main component of a sex pheromone that attracts ovulated females (Li et al. 2002). This pheromone is produced in the male liver and released from the gills, with a threshold of 10–12 M for female behavioral and olfactory responses. Small male sea lampreys produce higher rates of 3kPZS in the liver than larger males (Buchinger et al. 2017a). In many animals, males change their mating strategy depending on their body size to increase their mating success. In the sea lamprey, smaller males may release more sex pheromone than larger males to attract mating partners.
Previous behavioral studies showed the presence of unknown pheromonal compounds in addition to 3kPZS (Johnson et al. 2012), and several novel pheromones have been identified. For example, the bile alcohol 3,12-diketo-4,6-petromyzonene-24-sulfate (DkPES) was identified as a minor component of sex pheromones by bioassay-guided fractionation from water conditioned with spermiating male sea lampreys (Li et al. 2013). Ovulated females were attracted to artificial nests conditioned with a mixture of 3kPZS and DkPES at the male-released ratio of 30:1, respectively, and searching behavior in females in respond to the mixture was also observed, suggesting a function as a proximity pheromone (Brant et al. 2016b). Unsaturated bile salt 3-keto-1-ene-PZS was also identified as a sex pheromone from water conditioned with spermiating male sea lamprey; this compound attracted ovulated females to the spawning nests at a concentration of 10–12 M, equivalent to the effectiveness of 3kPZS (Johnson et al. 2014). Subsequently, three novel bile alcohols, all petromyzones, were isolated from water conditioned with spermiating male sea lamprey. The thresholds of these bile alcohols ranged from 10–11to 10–13 M in EOG recordings to ovulated females; One of these was attractive to ovulated females at 10–12 M and the others were not attractive or repulsive at 10–12 M to ovulated females, suggesting that these petromyzones may be putative pheromones in sea lamprey (Li et al. 2017). Recently, spermine in milt has been identified as a non-specific pheromone that stimulates ovulated female olfaction, activates TAARs in the olfactory epithelium, and attracts ovulated females at 10–14 M (Scott et al. 2019). Interestingly, semen in the herring Clupea harengus pallasi also induces the extension of urogenital papilla and the synchronization of spawning behavior in the reproductive season (Carolsfeld et al. 1997). Spermine induces olfactory responses in teleost fish (Rolen et al. 2003) and humans (Lefèvre et al. 2011) and is contained abundantly in semen (Tabor and Tabor 1984). The olfactory detection of semen may be conserved phylogenetically from jawless fish to vertebrates. These studies clearly indicate that female-attracting pheromone released from the male sea lamprey consists of multiple components. Even in sea lampreys in which male pheromones have been identified (see following paragraph), there is evidence that ovulated females release unidentified pheromone(s) that attract males (Teeter 1980; Siefkes et al. 2005).
The production of male lamprey pheromones is under the control of the endocrine system. The genes responsible for the biosynthesis and modification of bile salts were observed to be highly expressed in the liver and gills of the male sea lamprey (Brant et al. 2013). After reaching sexual maturity, sex pheromone-related bile acids seem to be biosynthesized dramatically in liver, transported to gills, converted enzymatically, regulated into a ratio of components, and released from the gill. In the transition from prespermiating to spermiating stage in the sea lamprey, progesterone acts within the testis to induce spermiation and also acts on both the liver and gill to promote the synthesis and release of 3kPZS (Bryan et al. 2015).
The priming effects of sex pheromones have also been studied in the sea lamprey. A single pheromone, 3kPZS, primes the secretion of 15α-hydroxyprogesterone in immature males but not in immature females, leading to activation of the hypothalamic-pituitary–gonadal (HPG) axis (Chung-Davidson et al. 2013b), while 3-keto-allocholic acid (3kACA) is an inhibitory pheromone that downregulates steroidogenesis in immature males within 24 h of exposure (Chung-Davidson et al. 2013a). Pre-ovulatory females and pre-spermiating and spermiating males primed by 3kACA and 3kPZS showed complex and differential changes in gonadal and plasma steroids (Chung-Davidson et al. 2020). Therefore, detailed investigations are needed to identify the pheromonal components and their pheromonal effects in the various developmental stages of reproductively receptive individuals.
The sex pheromones of sea lampreys appear to have evolved from their migratory pheromone cueing pathway (Buchinger et al. 2013, 2020; Brant et al. 2016a). Initially, 3kPZS was one of the by-products derived from bile acids in larvae of sea lamprey. Adult lampreys seemed to have evolved the ability to detect 3kPZS as an indicator of a suitable spawning site, with sexually mature males imitating larvae to release 3kPZS, resulting in deception of females. 3kPZS has been utilized as a male sex pheromone to attract ovulated females. In several lamprey species, partial overlap of male olfactory cues has been observed among intra- and interspecific variations of the bile acids produced as components in sex pheromones and migratory pheromones (Buchinger et al. 2017b, 2019). These findings may lead to elucidation of the evolution of sex pheromones in lampreys.
A brief description of the migratory pheromones, which are evolutionarily positioned as precursors of the sex pheromones in the sea lamprey, is also relevant in the context of this review. The first substance to be identified as a migratory pheromone in this species was a specific bile acid, petromyzonol sulfate (PZS), produced by larvae, which was investigated in detail from the viewpoint of olfactory electrophysiological responses (Li and Sorensen 1997). The threshold of olfactory responses was 10–12 M, suggesting that PZS binds to a specific receptor. Early-migrating adult animals, which are not yet ready to mate, respond to migratory pheromones. Larvae release PZS into the river via their feces, and adults respond to the migratory pheromones at early evening in laboratory and field experiments (Bjerselius et al. 2000).
In 2005, two novel bile acid sulfate conjugates (petromyzonamine disulfate [PADS] and petromyzosterol disulfate [PSDS]) were identified from about 8000 L of larval holding water. These migratory pheromones are released from the larvae and attract adult males and females. The thresholds of behavioral responses are 10–13 M for PADS and 10–11 M for PSDS, and the electrophysiological olfactory thresholds are both 10–13 M (Sorensen et al. 2005a; Fine and Sorensen 2008). However, some studies showed that these bile acid conjugates have a low activity. Subsequently, a novel pheromone was reported that resolved this issue: a fatty-acid derivative, (+)-petromyric acid [(+)-PMA], was identified by bioassay-guided fractionation as a migratory pheromone (Li K et al. 2018).
The first chemically identified sex pheromone candidate in fish is probably etiocholanolone (ETIO)-glucuronide, which in the black goby Gobius jozo is released by males to attract females (Colombo et al. 1980). In the round goby Neogobius melanostomus, reproductive males probably secrete female-attracting pheromones. The candidates for the active substances are ETIO, ETIO-glucosiduronate, 11-oxo-ETIO, and 11-oxo-ETIO-3-sulfate, as suggested by electrophysiological recordings from the olfactory epithelium of reproductive females (Murphy et al. 2001; Laframboise and Zielinski 2011), behavioral observations of responses to goby holding water (Belanger et al. 2004; Corkum et al. 2008), and biochemical analyses of the holding water (Arbuckle et al. 2005; Katare et al. 2011). Additionally, the round goby shows sexually dimorphic responses to some steroids, with males showing ventilatory behavior to estrone, E2-3β-glucuronide, and ETIO and females responding only to ETIO (Murphy et al. 2001). However, the biological function of these ventilation responses is not known well.
In the yellowfin Baikal sculpin Cottocomephorus grewingki, mature males release sex pheromones to attract mature females and induce female maturation. The candidates are testosterone, 11β-hydroxytestosterone, and 2Z,6E-farnesol in the male urine (Katsel et al. 1992). In related species, increased urine release and hypertrophied kidney were found to be associated with nesting behavior or sexual maturation in the male freshwater sculpin Cottus pollux small-egg type (SE) (Koya et al. 2015, 2016). These findings suggest that male urine of the freshwater sculpin contains female-attracting pheromones during the spawning season. According to this hypothesis, in the spawning season, male fishes with hypertrophied kidney would secrete male pheromones that attract females, as also suggested in sticklebacks managing their nests (Mclennan 2003, 2005). Male pheromones in these species have not yet been chemically identified.
Sex pheromones were investigated in the African catfish Clarias gariepinus with the overall aim to improve the aquaculture of this species (Lambert and Resink 1991). In this study, a mixture of seven steroid glucuronides contained in the male’s seminal vesicles seemed to attract ovulatory females, and another pheromone released from mature males likely primed their ovulation. Among these seven glucuronides, the most potent odorant in female olfaction was 3α,17α-dihydroxy-5β-pregnan-20-one-3α-glucuronide. Further studies with a specific behavioral assay are needed to test these pheromone candidates.
Tilapia, which are cultivated in hot pools at high latitudes or which are invasive in various warm-water areas, have been studied with respect to male urinary pheromones. In a study carried out on the Mozambiqu tilapia Oreochromis mossambicuse, EOG recordings showed that females are sensitive to conspecific male bile, feces, and urine (Frade et al. 2002), while males can discriminate between pre- and post-ovulatory females via both the urine and feces and can enhance their urination rate in the presence of pre-ovulatory females (Miranda et al. 2005). These findings suggest the presence of chemical cues from pre-ovulatory females.
Dominant males of the Mozambique tilapia are able to store a large amount of urine, and urine can be actively released during aggressive encounters (Barata et al. 2007). The olfactory sensitivity of pre-ovulatory females to the urine increases according to male social hierarchy, suggesting that odorants in dominant male urine (DMU) act as a dominance or sex pheromone. An aminosterol was isolated from DMU of the Mozambique tilapia (Barata et al. 2008), and a male-specific protein similar to lipocalin was identified in DMU of other tilapia species (Machnes et al. 2008). Dominant males have large urinary bladders with developed detrusor muscles and urinate frequently when their androgen levels are high and/or when subordinate males are in the same tank (Keller-Costa et al. 2012). EOG recordings suggest that the male pheromones affecting preovulatory females are 5β-pregnane-3α,17α,20β-triol 3-glucuronate and its 20α-epimer (20α- and 20β-P-3-G), and that a putative female pheromone affecting mature males is 17β-estradiol-3-glucuronide (E2-3 g) (Keller-Costa et al. 2014b, 2014a). Indeed, a mixture of the synthetic compound 20α/β-P-3-G, identified from DMU evoked priming effects that showed drastic increases in female 17,20β-P (maturation-inducing hormone) 1 h after exposure (Huertas et al. 2014; Keller-Costa et al. 2014a).
DMU of the Mozambique tilapia contains not only female primer pheromones but also a conflict-inhibiting pheromone, composed of both polar and non-polar chemicals, which reduces male–male aggression (Keller-Costa et al. 2016). DMU also increases the release rate of 11-ketotestosterone in subordinate males but reduces male–male aggression, suggesting that DMU may act in chemical diplomacy (Saraiva et al. 2017).
In the Arctic char Salvelinus alpinus, PGFs derived from mature males were reported to show releaser effects that induce attraction and nesting behavior in females, but do not attract males (Sveinsson and Hara 1995). However, many questions remain regarding the levels of the secreted PGFs, the timing of the release by males, and the origin of the PGFs. The olfactory organ of the lake char Salvelinus namaycush is sensitive to bile acids (Zhang et al. 2001; Zhang and Hara 2009). Male and female lake char are attracted to water conditioned by males or juveniles (Buchinger et al. 2015). In the Arctic char, a pheromone hypothesis of homing migration—that the odor of young fish attracts adult fish to the natal river—has long been postulated (Nordeng 2009). Inferring from the above, Arctic char, which is a relatively ancestral salmonid, is thought to have a complex chemical communication system by secreting or receiving chemical cues not only in mature fish but also in immature fish. Based on its life-cycle, Salvelinus species may belong to a genus that is highly dependent on its chemical senses.
Even in zebrafish, in which female pheromones have been identified (see above text), steroid glucuronides such as 5α-androstane-3α,17β-diol-glucuronide from mature male holding water have been suggested to stimulate female ovulation (Van Den Hurk et al. 1987). This suggestion has been reiterated in recent years, i.e. that male pheromones are able to induce ovulation and attract females (Li J et al. 2018).
Male pheromones have been reported in species in which the male determines the nesting or spawning sites. Since females generally have fewer gametes than males, as mentioned in sea lamprey, males may release many components of pheromones. Males probably need multiple components as signals to represent male abilities, physiological and health conditions because females are more severe in their mate choice than males.
Application of fish sex pheromones
Sex pheromones are a very interesting research area, not only in terms of reproductive biology but also for exploring reproductive isolation mechanisms, including speciation. Alternatively, in terms of practical applications, the sex pheromones of insects have long been applied in pest control programs; for example, traps baited with pheromone are used to capture and kill pests, or the release of a large amount of pheromone into the local environment is used to disturb the chemical communication of pests. In recent years, therefore, pheromone-utilized fishing methods are also expected to be put into practice for sex-selective fishing or in extermination methods that do not adversely affect the natural environment.
Pheromone traps are a promising application of fish pheromones. Sea lampreys have long been dealt with as invasive organisms in the Great Lakes, and the results from applied research using pheromones have been active in developing methods to help exterminate them. One example of such control efforts is to increase the number of unfertilized eggs in the population, which is expected to reduce the size of the population (Siefkes et al. 2003). In this approach, after confirmation of sex pheromone production in spermiating males sterilized by a drug, the sterilized males are released into rivers to mate with females with the expectation that the ovulated eggs will be fertilized with sterilized semen and not develop. A second method of controlling sea lampreys is by using a pheromone trap that directly exterminates adults. In field experiments using migratory pheromones and sex pheromones, both sexes of migratory adults and ovulated females were efficiently captured by pheromone traps (Wagner et al. 2006). 3kPZS was especially effective as bait in sex pheromone traps for attracting ovulated females from up to several hundred meters downstream of the source, and the pheromone in the trap was as active as the natural pheromones released from spermiating males. In one trap experiment, a high concentration of 3kPZS was more attractive than male-conditioning water (Johnson et al. 2009). In another study, trap tests with synthetic 3kPZS were performed in eight Michigan streams over 3 years (Johnson et al. 2013). In terms of overall yearly trapping efficiency, there was about a 10% higher level during years when 3kPZS was introduced. The most suitable conditions for pheromone traps with 3kPZS are wide streams, a low abundance of adult sea lampreys, and nights early in the spawning season (Johnson et al. 2015). A polyethylene glycol matrix was developed as a cost-effective emitter to release 3kPZS from pheromone traps into rivers (Wagner et al. 2018). Methods for using 3kPZS to trap invasive sea lampreys have been developed that take into account biological and environmental conditions, such as sea lamprey length, sex, water temperature, and stream width (Johnson et al. 2020). Not only sex pheromones but also migratory pheromones have been applied as tools for management of the sea lamprey. Unnatural pheromone analogs of PZS produced by chemical synthesis have been shown to activate the olfactory pathway of sea lampreys and have the potential to be used in their control (Burns et al. 2011). Although migratory pheromone blends containing unknown components have been suggested in previous reports, even partial pheromones composed of PADS and PSDS facilitate adult lamprey to approach river entrances in the absence of a complete blend of pheromones (Meckley et al. 2014). Pheromone traps are practical tools for management of the sea lamprey because this species highly depends on olfaction and has multiple pheromones. The use of pheromone traps to control invasive sea lampreys has been reviewed in detail in terms of practices, strategies, registration, and application over 10 years (Li et al. 2007; Sorensen and Johnson 2016; Siefkes 2017; Fredricks et al. 2020). Given that the social behavior of primitive vertebrates, such as the sea lamprey, is strongly dependent on chemical senses and that the pheromones of the lamprey were identified using wild populations, the application of pheromones may be effective strategy to control the wild populations. In contrast, in some of other fish species, the pheromones were identified using cultured populations derived from a few families; in these cases, the pheromone (blend) identified might be different from that of the wild population and the effect of the identified pheromone (blend) on the wild population might be lower. Fish species that depend on visual and auditory senses in social interactions also may not respond in the same manner to pheromones as does the sea lamprey.
The largemouth bass Micropterus salmoides is one of the most invasive fish species worldwide and has a considerable impact on the diversity of aquatic communities. Therefore, many projects aimed at controlling largemouth bass populations have been conducted in Japan (Wildlife Division 2004). In this context, pheromone trapping has been studied as a means to effectively control populations of largemouth bass. In the spawning season, reproductive males make nests in the bottom sediment for female spawning. In one study, when bile from reproductive male (RB) largemouth bass was introduced into the traps, the number of mature females captured by the trap was significantly higher than when bile from non-reproductive males was used as bait; other fish species did not show any preference for RB (Fujimoto et al. 2020). These results showed that reproductive male bile contains female-attracting pheromone(s), suggesting that pheromone traps have the potential to be used to control the populations of invasive largemouth bass.
The common carp Cyprinus carpio has been recognized as an invasive fish in North America. Common carp injected with PGF2α release metabolites of PGF2α as sex pheromones, and these attract conspecific males in both the laboratory and field tests (Lim and Sorensen 2012). PGFs as sex pheromones have also been used to determine the reproductive condition of invasive common carp, using environmental DNA to estimate the population sizes (Ghosal et al. 2018).
Fish pheromones could also be useful in aquaculture. It may be possible to use the priming effects of sex pheromones to synchronize ovulation and spermiation in fish farms and hatcheries, based on the observation that the odors from mature members of the opposite sex prime not only the secretion of sex steroids and the gonadal development in many species but also spermatogenesis in immature European eel Anguilla anguilla L. males (Huertas et al. 2006). Priming effects may increase the expressible milt in mature male fish that have a small testis, such as the huchen Hucho perryi. In rearing ponds, introducing sex attractants into separated areas may prevent males or females from overmaturation through segregating the two sexes, as has been suggested conventionally in salmon hatcheries for a long time (Yambe, unpublished observation).
Thus, sex pheromones are expected to be applied to invasive species and commercially important species. The application of the non-hormonal pheromone carried a relatively low risk because it shows a high species specificity and non-target species do not respond behaviorally and physiologically to it. In contrast, hormonal pheromones may disrupt mating behavior and the endocrine system in many vertebrates, including fish, because multiple animals respond to the same molecules, such as PGFs and steroid hormones. Thus, the field applications of pheromones need to be considered carefully.
Chemical cues in aggregation and habitat selection
Habitat selection is critical for organisms to obtain their food, find refugee from predation, and find suitable places for reproduction. The presence of successful conspecifics indicates a preferable habitat. Thus, planktonic motile larvae of immotile benthic organisms settle around conspecifics. Aggregation also benefits the animals in some ecological situations, such as by avoiding predation and finding food (Allee 1927; Davies et al. 2012). Aggregation or accumulation of individuals is also necessary for finding mates.
Fish schooling behavior is partially mediated by chemical cues. The eeltail catfish Plotosus japonicus (formerly Plotosus lineatus) (Yoshino and Kishimoto 2008), which is called “Gonzui” in Japanese, forms a dense school immediately after hatching, and school recognition is under the control of chemical cues emitted by the school members. The key substances governing this recognition in the eeltail catfish have been deduced to be a mixture of phosphatidylcholines (PCs), and modification of the natural PC profile disrupts the recognition of the school odor (Matsumura et al. 2004, 2007). The PC profile may change depending on genetic differences and feeding history. The members of a school can share these factors by being siblings and by foraging together since being hatched from the egg. This type of chemical cue, signature mixtures, is the basis of individual recognition based on learning the different chemical profiles of individuals, allowing familiar and unfamiliar animals to be distinguished (Wyatt 2014).
In spiny lobster P. argus fisheries in the US state of Florida, fishermen use small-sized spiny lobsters, called “shorts,” as bait in lobster traps to attract larger legal-sized lobsters (Hunt 2000). This attraction is based on aggregation behavior using chemical cues present in the urine of the lobster (Horner et al. 2006, 2008; Lozano-Álvarez et al. 2018). However, the shorts used in the trap die in the trap, leading not only to a decrease in the wild lobster population (Butler et al. 2018a) but starvation in the trap also to a reduction in their attractiveness as live bait (Butler et al. 2018b). Therefore, artificially prepared attractants should replace the shorts to conserve lobster populations, but the attractants released from short spiny lobsters have not yet been identified. Fisheries in the USA utilize chemical communication to catch crustaceans, the blue crab C. sapidus and the spiny lobster P. argus.
Chemical cues that mediate larval settlement have been reviewed by Harder et al. (2018). Invertebrate larvae find their preferable substrate to settle by chemical cues from conspecifics and other species. Generally, the substrates identified as a natural source of larval settlement cues are mixtures of multiple species of micro- and macro-organisms (e.g., natural biofilms, algae, and body surface of animals). One of the most clearly identified conspecific settlement cues is the settlement‐inducing protein complex (SIPC) in barnacles, which is released from adults and induces larvae to settle around the adults (Matsumura et al. 1998). Crustose coralline algae cell wall-associated compounds, namely, glycoglycerolipids and polysaccharides, have been identified as the main constituents of the settlement cues of corals. These algae-derived fractions induce settlement and metamorphosis in ecologically relevant conditions (Tebben et al. 2015). The Barnacle settlement cue, SIPC, is an example of a relatively simple combination of molecules working as a settlement cue; such cues from other species seem to be more complexed, and it is likely that many of the as-yet unidentified cues are mixture of compounds.
The homing substance for homing to a natal river in anadromous salmon is one of the most remarkable chemical cues in fish life-history (Bett and Hinch 2016). There are two principal hypotheses for the chemical cues used in this homing behavior: imprinted natal substances (Wisby and Hasler 1954) and pheromonal substances (Vrieze et al. 2010). The olfactory imprinting and homing mechanisms have been studied using behavioral, electrophysiological, biochemical, and neurobiological methods (Ueda 2011), with the aim to substantiate the olfactory hypothesis based on natal stream odor, which was proposed in the coho salmon Oncorhynchus kisutch (Wisby and Hasler 1954).
The olfactory organs of both the lacustrine sockeye salmon and masu salmon respond differentially to various freshwaters, regardless of sex or gonadal maturity (Sato et al. 2000). According to studies showing the similarity of olfactory responses to artificial stream water based on the composition of dissolved free amino acids (DFAAs) and the corresponding natural stream water, amino acids dissolved in the natal stream water are thought to be the natal stream odors (Shoji et al. 2000). Additionally, Y-maze tests demonstrated that artificial home stream water that contained these DFAAs was selected by mature male chum salmon Oncorhynchus keta (Shoji et al. 2003). In another study, after incubating the biofilm of stones, which had been placed in a river, for 24 h at stream water temperature, DFAAs in the incubation solution were analyzed by HPLC, and the results suggested that the biofilms are a major source of DFAAs in stream water (Ishizawa et al. 2010). The salmon olfactory imprinting-related gene (SOIG) of lacustrine sockeye salmon, which was identified by the subtractive hybridization technique of cDNA-representational difference analysis (cDNA-RDA), might be related to olfaction during both the parr–smolt transformation (PST) and the final stage of homing (Hino et al. 2007). In lacustrine sockeye salmon, the imprinting of natal stream odors in juveniles and homing migration in mature salmon were shown in electrophysiological and behavioral experiments. Mature salmon that were exposed to an amino acid for 2 weeks during PST 2 years prior to the experiment showed a preference behavior and a remarkably high olfactory response to the amino acid (Yamamoto et al. 2010). Several Oncorhynchus species appear to imprint not only to amino acids but also to morpholine and phenethyl alcohol, which are not found in natural river water (Bett and Hinch 2016). In four Oncorhynchus species, behavioral responses to artificial natal stream water (ANW), which was prepared to have the same composition and concentration of DFAAs as in their natural natal stream, were observed in Y-maze tests. Chum, sockeye, and masu salmon preferred the ANW to controls, whereas the pink salmon Oncorhynchus gorbuscha showed the lowest selectivity and the highest locomotive behavior to ANW (Yamamoto et al. 2008). These results may suggest an evolutionary tendency in salmonids because species such as pink salmon that are more dependent on the ocean, traveling to sea immediately after hatching, are less dependent on the natal river.
Unlike Salvelinus species, Oncorhynchus species are not thought to use pheromones emitted by juveniles in homing. However, sockeye salmon are attracted to conspecific odors when imprinted natal cues are absent, but not when they are present, implying that pheromones may provide a directional cue that is secondary to the primary cue of imprinted chemicals (Bett and Hinch 2015). Specifically, for homing in adult salmon, if something is wrong with the odor of the natal stream, the conspecific odor will be utilized as secondary chemical cues.
Recognition of diseased conspecifics using chemical cues
The Caribbean spiny lobster P. argus uses chemical cues to detect and avoid shelters containing conspecifics infected with the PaV1 virus. The chemical cues are contained in the urine, and spiny lobsters can avoid infected animals effectively in low-flow regimes (Behringer et al. 2006; Anderson and Behringer 2013; Lozano-Álvarez et al. 2018; Ross and Behringer 2019). Chemical cues for parasite avoidance have remained important throughout the evolutionary history of vertebrates, including fish and amphibians. For example, rainbow trout infected with the trematode Diplostomum sp. release chemical alarm substances that increase the activity of unexposed conspecifics. Similarly, bullfrog tadpoles Rana catesbeiana use chemical cues from conspecifics to avoid infection by the pathogenic yeast Candida humicola (Kiesecker et al. 1999). It is possible that the molecules indicating infection can be identified by comparing the body odor or urine of healthy and diseased animals. These molecules that induce avoidance behavior can be used to repel animals from unfavorable locations in aquaculture tanks, such as the water outlet(s) of a tank.
Chemically stimulated grooming behavior
Many crustacean species groom their body parts to remove epibionts to avoid fouling (Bauer 1977, 1981). In one study, tidepool shrimps Heptacarpus pictus prevented from antennule grooming by ablation of the third maxillipeds suffered from fouling by epibionts on the antennule (Bauer 1977). Phyllosoma larvae of the smooth fan lobster Ibacus novemdentatus that stay and feed on jellyfishes groom their body by elongated third maxillipeds; phyllosoma prevented from grooming by ablation of third maxillipeds had increased fouling by epibionts (Kamio et al. 2015). The grooming behaviors consist of auto grooming and induced grooming elicited by chemical cues. Interestingly, antennule grooming behavior of the Caribbean spiny lobster P. argus is elicited primarily by l-glutamate (Barbato and Daniel 1997), and non-olfactory chemoreceptor neurons in asymmetric sensilla activate the grooming behavior (Schmidt and Derby 2005). Secretions produced by the tegumental glands surrounding the base of aesthetasc sensilla might provide chemical antifouling agents and/or substances that aid in the stabilization/protection of the extremely delicate cuticle of the aesthetasc (Schmidt and Derby 2005). l-Glutamate also elicits grooming behavior of the phyllosoma in the smooth fan lobster (Kamio et al. 2015). Grooming behavior seems to be associated with the secretion of antifouling molecules, and these molecules are candidate antifouling agents.
Parasites: host recognition using chemical cues
Parasitic animals use chemicals cues to find hosts and to invade and find places to settle in the body of the host (Haas 2003). Entomopathogenic nematodes find and select their insect host by sensing CO2 and host odorant mixtures (Dillman et al. 2012). In aquaculture environments, a parasitic copepod, the sea louse Lepeophtheirus salmonis, which specifically parasitizes salmonid species, uses chemical cues from their host (Mordue Luntz and Birkett 2009). Compounds identified from salmon-conditioned water extracts (e.g., isophorone [6-methyl-5-hepten-2-one] have been shown to switch on positive rheotaxis (upstream orientation in flowing water) in adults and copepodids (Ingvarsdóttir et al. 2002; Bailey et al. 2006). In copepodids, the presence of chemicals such as 4-methylquinazoline and 2-aminoacetophenone from a non-host species, the turbot Psetta maxima, prevents the normal positive rheotactic movement of copepodids to salmon-conditioned water when both are presented together in a Y-tube olfactometer (Bailey et al. 2006). The pheromone can be used to eliminate the copepods by attracting them and/or disrupting their mating behavior.
The nemertean worm Carcinonermertes errans is a parasitic egg predator of the Dungeness crab Metacarcinus magister. In laboratory experiments, larvae of the parasitic worm preferentially settled on the exoskeleton of the crab and migrated under its abdominal flap where they incubate their eggs. In field studies, competent larvae of the worm present in the waters could infect crab hosts directly from the water column, and exhibited density-dependent gregarious settlement (Dunn and Young 2014). These results indicate the release of chemical cues from the host.
The rhizocephalan barnacle Loxothylacus texanus parasitizes the blue crab C. sapidus, detecting chemical cues to settle on the crabs. This parasite severely impacts crab fisheries because the gonads of parasitized crabs do not mature and, consequently, these crabs do not reproduce and male crabs are feminized; in addition, the molt ceases before the crabs reach legal size. The cypris larvae of L. texanus settle in response to carbohydrate or glycoprotein cues found in the epicuticle layer of the C. sapidus exoskeleton and only settle on intermolt epicuticle but not on the newly generated internal cuticle. Evolutionarily, this type of specificity may minimize premature settlement on an unsuitable substrate, such as a dead or partially eaten crab (Boone et al. 2003). Settlement cues for these parasites might be used as agents for parasite control.
Finding and selecting food
Marine organisms use their chemical senses to find and select food (Kamio and Derby 2017). Therefore, understanding how chemical cues are used in food detection is useful for developing artificial food and attractants in fisheries and aquaculture (Kamio and Derby 2017). In aquaculture, the nutritional value of the food provided is important for improving the health and growth of animals (Takeuchi 2014; Takeuchi and Haga 2015; Okamura et al. 2020), but the food must be both attractive to and ingested by the animals. Chemosensory biology can be used to develop effective feeding. Amino acids, organic acids, and nucleic acids have been identified as feeding stimulants in marine animals (Carr and Derby 1986; Carr 1988). Betaine, taurine, trimethylamine oxide, glycine, alanine, proline, homarine, and arginine are typically the most abundant compounds in mollusks and crustaceans. Among these, glycine, alanine, proline, arginine, and betaine are stimulants for fish. Other minor components are also important stimulants for some fish species (Carr et al. 1996).
The feeding mechanisms of the Pacific white shrimp Litopenaeus vannamei have recently been studied with the aim to improve the quality of food in the aquaculture of this shrimp. Litopenaeus vannamei is often grown on feed containing 30–40% soybean meal or other sources of plant protein because of the favorable cost and availability of plant protein compared to marine animal protein. Some experiments have used krill meal as a supplement to improve the quality of feed containing soybean meal. Krill meal is a chemostimulant whose major effect when added to feed pellets is to increase the pellets’ palatability by prolonging the feeding session and thus the amount eaten, but not affecting how quickly a shrimp eats each pellet (Derby et al. 2016b). Using this experimental method, a highly effective chemostimulant without animal products was developed. This artificial chemostimulant is more attractive and palatable than krill meal and, consequently, the growth rate of the shrimp increased when this artificial chemostimulant was used (Derby et al. 2018). Another chemostimulant that enhances the palatability of feed pellets was also developed using the same approach (Morais and Derby 2019). The chemosensory control of feeding in L. vannamei is generally similar to that in the better-studied crustaceans, including spiny lobsters (Achelata), clawed lobsters and crayfish (Astacidea), and crabs (Meirua). Since L vannamei is important as the principal species in the worldwide aquaculture of shrimp and it has also become a model in the study of crustacean biology as one of the first decapod crustaceans to have its genome sequenced, the morphology of chemosensory organs and their control of chemosensory behaviors in feeding have been described in detail (Dana et al. 2020).
The aquaculture of phyllosoma of the slipper lobster I. novemdentatus grown on jellyfish (Kamio et al. 2016b; Wakabayashi et al. 2019) requires artificial food because the jellyfish supply mainly depends on wild populations and is not stable. Addition of the amino acid glycine to a urethane sponge stimulates predatory behavior in the phyllosoma (Kamio et al. 2015). Other unknown molecules might also increase the attractivity and palatability of artificial food.
Artificial chemoattractants could be applied to commercial fishing using baited traps. As an attractant in basket traps for blue swimmer crab Portunus armatus (formerly Portunus pelagicus) and Asian paddle crab Charybdis japonica, a bait combination of sugarcane and fish was found to be more effective than fish bait alone, whereas sugarcane alone was ineffective. The use of this sugarcane–fish combination resulted in an extremely male-biased catch of blue swimmer crab; this result would have a positive effect on the conservation of the population of this crab (Kawamura et al. 1995) given that the remaining males would have enough sperm to fertilize all eggs in a female-biased population (but see the discussion on sperm limitation in Sato and Goshima 2006). However, in an experiment in which purified sugars were used as trap bait, the catch in several crab species, including T. prymna and C. japonica, decreased after sugar had been added to the mince when compared to “fish” bait, possibly because refined white sugar lacks many of the unidentified attractants present in sugarcane (Archdale et al. 2008).
Thus, there are chemical cues that can enhance feeding and can attract specific organisms selectively. Commercially available food and baits can be designed to be effective, but there is a potential to be more efficient by controlling chemical cues contained in the food and baits.
Rheological environment in benthic foraging
Environmental water flow affects feeding success in the field (Weissburg 2012). For example, the blue crab C. sapidus finds clams more efficiently in smooth-turbulent flows than in rough-turbulent flows in flume experiments (Weissburg and Zimmer-Faust 1994). Callinectes sapidus can distinguish and navigate to food odors even when aversive odors (injured crab metabolites) are released nearby. However, environmentally produced turbulence suppresses tracking by homogenizing the two odors, indicating that mixing in the natural environment may amplify the effects of predators by suppressing tracking to food odors when aversive cues are present (Weissburg et al. 2012). In contrast, the whelk Busycon carica hunts successfully even when bottom roughness increases the turbulent mixing of prey chemicals (Ferner et al. 2009) because it can continue pursuing prey in areas where odors are rapidly mixed and diluted (Ferner and Weissburg 2005). Laboratory experiments have shown that water flow can influence the feeding success of animals in aquaculture tanks. Environmental water flow can boost foraging success of the juvenile rapa whelk Rapana venosa in aquaculture tanks (Yu et al. 2019). Thus, it is important to apply the rheological conditions specific to each species to achieve successful feeding in aquaculture and trapping using bait in fisheries, particularly for slow-moving benthic animals such as the rapa whelk.
Chemical cues that suppress activity of animals
Predators influence the behavior and physiology of prey without physically attacking and damaging them. These are called nonconsumptive predator effects, and one of the cues that affects prey is the odor of predators (Weissburg et al. 2014). The odor of the predatory European green crab C. maenas reduces movement and foraging activity of the prey dogwhelk Nucella lapillus (Large et al. 2011). Odors from the predatory blue crab C. sapidus and knobbed whelk B. carica suppress pumping by the hard clam M. mercenaria (Smee and Weissburg 2006). The odor of predators affects the growth of prey, providing them with greater protection. Exudates from the predatory blue crab induce stronger effects in the eastern oyster Crassostrea virginica compared to those from the scavenging blue crab. This stronger response to a predator than to a scavenger could be due to inherent differences in diet cues representative of reduced risk in the presence of scavengers or to degradation of conspecific alarm cues in aged treatments, which may mask the risk from potential predators subsisting by scavenging (Sherker et al. 2017).
The source and identity of predator odor molecules were studied in a predatory–prey system involving the blue crab and the mud crab Panopeus herbstii. Mud crabs respond to the scent of predatory blue crabs by reducing their activity, a form of alarm behavior (Hill and Weissburg 2014). The feeding suppressant is present in the urine (Weissburg et al. 2016). Using NMR spectroscopy and MS-based metabolomics, the chemical variation in urine from blue crabs fed different diets was related to prey behavior. The urinary metabolites trigonelline and homarine were identified as components of the odorants that mud crabs use to detect blue crabs (Poulin et al. 2018).
Fish also avoid predatory and alarm cues. Frisch (1938; 1942) reported that injured skin of the minnow Phoxinus laevis releases a substance that evokes fear in conspecifics. Subsequently, hypoxanthine-3-N-oxide (H3NO) was identified as the putative alarm pheromone of ostariophysan fishes, and the fathead minnow Pimephales promelas learned to recognize predator odors when exposed to H3NO (Brown et al. 2001). H3NO together with other N-oxides and conspecific skin extract elicit significant increases in antipredator behaviors in the juvenile channel catfish (Brown et al. 2003). The rainbow trout shows antipredator responses to conspecific skin extract and predator odor (Brown and Smith 1997; 1998). The sea lamprey avoids odors that represent danger in their habitat, conspecific alarm cues, and predator cues such as 2-phenylethylamine hydrochloride (PEA HCl), which is found in the urine of mammalian predators (Di Rocco et al. 2015).
Animals stop feeding when they sense odors from threatened conspecifics. The blue crab decreases foraging activity in the presence of odors released from freshly injured conspecifics that indicate the presence of predators, as shown in the field (Ferner et al. 2005) and laboratory (Moir and Weissburg 2009). Injured Caribbean spiny lobsters passively release alarm cues via their hemolymph (Shabani et al. 2008; Lozano-Álvarez et al. 2018). Hemolymph suppresses the food searching responses of lobster, and the alarm cue is detected by olfactory receptor neurons in the aesthetasc sensilla (Shabani et al. 2008). These alarm molecules in the hemolymph have not yet been identified and are possibly species-specific chemical cues.
The sea hare Aplysia californica releases ink and opaline when attacked by predators, and both secretions contain alarm cues. The alarm cues in the ink are uracil, uridine, and cytidine (Kicklighter et al. 2007), and those in opaline are three mycosporine-like amino acids (MAAs) (Kamio et al. 2011; Kicklighter et al. 2011). The effect of ink and opaline on the feeding behavior of conspecifics was evaluated by measuring the number of ingestive radular movements (Wolfe et al. 2016). In that study, ink suppressed feeding behavior immediately, but the effect was short-lived (15 min). Since the sea hare possesses chemical defenses to defend against consumers, it is perhaps unnecessary or too costly to maintain feeding suppression after the risk subsides.
Thus, stressful chemical cues are recognized in broad taxa of aquatic organisms, including fish (Reverter et al. 2018) and polychaetes (Schaum et al. 2013). These chemical cues of danger suppress various activiies of animals, such as foraging, and thus decrease productivity in aquaculture systems. The management of stress in animals is important for aquaculture, and methods for detecting such stress is an active area of research (Endo and Wu 2019). Management of these stressful odors may keep organisms in aquaculture facilities in a healthy condition, thus increasing production. If an animal in a crab pod happens to be injured, the other crabs may be repelled, resulting in low productivity of the crab pod. In contrast, cues of danger can be used as a repellent to avoid unwanted animals.
Chemical defense
Defensive molecules released from organisms can be received as chemical cues by consumers or sessile organisms, leading to unsuccessful feeding and settlement. The use of distasteful molecules, called deterrents, is a broadly recognized chemical defense mechanism that reduces the feeding activity of consumers of plants (Hay and Fenical 1988), invertebrates (Pawlik 1993), and fish (Tachibana et al. 1985). The ejection of appetitive molecules that stimulate food searching as a defense behavior, called phagomimicry, is known for a few species of mollusks (Derby et al. 2007; Derby 2014). The sea hare A. californica employs both distasteful and tasteful chemical defense cues. This marine snail has defensive glands in its mantle cavity, namely, the ink gland and opaline gland (Johnson et al. 2006). Deterrents are contained in both the purple ink secretion and the white opaline secretion. Aplysioviolin and phycoerythrobilin have been identified as deterrents in the ink secretion (Kamio et al. 2010a). These molecules are synthesized from food derived from the photosynthetic light-harvesting protein phycoerythrin (Kamio et al. 2010b). Aplysia californica produces deterrent molecules outside of their body upon attacked by a predator. The ink secretion contains an l-amino acid oxidase, escapin (Yang et al. 2005), and opaline contains its substrate, l-lysine (Johnson et al. 2006). The resultant enzymatic reaction produces hydrogen peroxide, ammonia, and an equilibrium mixture that starts with α-keto-ε-aminocaproic acid (Kamio et al. 2009), with the aim to suppress the feeding behavior of crabs and fish (Kamio et al. 2010a; Nusnbaum and Derby 2010). The ink and opaline secretions contain high levels of tasteful amino acids, and this secretion can induce feeding behavior in the Californica spiny lobster Panulirus interruptus to facilitate the sea hare’s escape from predation (Kicklighter et al. 2005). Sea hares also release defensive chemicals from their skin when they are attacked by predators. The walking sea hare Aplysia juliana, which lacks purple pigment in its ink, has deterrents in its skin rather than in the ink (Hayashihara and Kamio 2016; Kamio et al. 2016a). Many other bioactive molecules are found in sea hares in the ecological and biomedical context (Kamiya et al. 2006). Thus, sea hares, including A. californica, are interesting animals because they are equipped with diverse chemical defense mechanisms. The type of chemical defense of this species can be explained as follows. Iits antipredator chemical defense acts on the chemical senses of the predator in diverse ways, namely, as a deterrent, a feeding stimulant, and a mixture of both. The defensive molecules are stored as is or produced when the prey is attacked by a predator. Some of the defensive systems depend on their food-algae-derived compounds and others do not. These defensive systems could be utilized as repellents in the aquatic environment.
Chemical defenses could be effective without being cues detected by the chemosensory systems of consumers and without inhibiting the consumers’ feeding. These chemical defenses work by lowering the fitness of the consumers and thus affecting the consumers’ population size. Young leaves of the tropical forest tree Inga umbellifera contain high levels of l-tyrosine. Artificial food containing relevant levels of l-tyrosine inhibit the growth of larvae of the noctuid moth Heliothis virescens (Lokvam et al. 2006). The Antarctic sponge Isodictya erinacea produces a tryptophan catabolite, erebusinone, that causes significantly reduced molting and proportionally increased mortality at ecologically relevant concentrations when fed to sympatric predatory amphipods (Moon et al. 2000). These molecules are very harmful because animals cannot avoid it by detecting as unfavorable molecules through their chemosensory systems. These molecules also cannot be found in experiments guided by feeding behavior.
Chemical defense can also be used as an antifouling mechanism. Marine organisms protect their body surface from fouling by microorganisms, bacteria, and microalgae that develop biofilms, and from macroorganisms, barnacles, mussels, polychaetes, and macroalgae using physical (Scardino and de Nys 2011) and chemical defenses (Fusetani 2004). New natural products that have antifouling activity have been reported (Almeida and Vasconcelos 2015; Qian et al. 2015; Liu et al. 2020). For example, effective antifoulants against barnacles and microfoulants (Oguri et al. 2017) have been found in the red alga Laurencia sp. (Oguri et al. 2017), tunicates (Levert et al. 2020), the mollusk Phidiana militaris (Levert et al. 2020), holothurians (Kamyab et al. 2020), and sponges (Tintillier et al. 2020). Antifoulants may improve the success of marine invasive tunicates (Utermann et al. 2020; Nagai et al. 2021).
Antifouling is enhanced by combining the physical and chemical characters of biological systems (Chapman et al. 2014). Future discoveries of natural antifoulants from marine organisms have the potential to aid in the development of environmentally friendly antifouling devices (Oguri et al. 2017; Al-Lihaibi et al. 2019).
Global issues
Prediction of the influence of anthropogenic global changes on the chemoreception of aquatic organisms is an active research field, particularly on the topics of behavioral responses to ocean acidification and petrogenic plastic pollution. Studies on these chemically mediated interactions among organisms provide insights into the ecology and evolution of marine communities (Hay 2009), including the effects of ocean acidification on marine communities (Wyatt et al. 2014; Del Monaco et al. 2017) because changes in pH may modify the shape of molecules and receptor proteins (Roggatz et al. 2016). Fluctuations in the pH of water affect the chemosensory behaviors of fresh water and marine organisms. Chemical communication, parental care, mate selection, habitat selection, including homing and settlement, and predator–prey interactions affecting the chemosensory ability of both prey and preditor can be affected by increases or decreases in pH (Kitamura and Ikuta 2000; Heuschele and Candolin 2007; Leduc et al. 2013; Ashur et al. 2017). This also means that organisms used in fisheries and aquaculture will be affected by pH fluctuations in environmental water.
Plastic debris, especially microplastics, have been found worldwide in all marine compartments and is increasing in volume (Isobe et al. 2019). These plastics release chemicals that are assumed to affect the chemosensory behavior of organisms; for example, the sea anemone Exaiptasia pallida has been found to ingest plastic pellets (Diana et al. 2020). Plastic debris adsorbs hydrophobic organic compounds from the surrounding water (Mato et al. 2001) and accommodates microbial communities on its surface (Zettler et al. 2013) that release metabolites. Plastic debris colonized by microbials emits a keystone molecule dimethyl sulfide that is a feeding attractant for olfactory foraging seabirds and many other organisms, and the debris is thus eaten by the consumer as wrong food (Savoca et al. 2016).
Plastic additives that are incorporated into plastics can leach out as most of these are not chemically bound (Hermabessiere et al. 2017). Compounds leached from polystyrene pellets impair the vigilance and avoidance behavior of the intertidal gastropod Littorina littorea to the predatory chemical cue of the European green crab C. maenas (Seuront 2018). Hard corals ingest various types of plastics but not sand, indicating that phagostimulants are leaching out from the plastics (Allen et al. 2017). One category of plastic additive, phthalate esters, is reported as natural products of bacteria and algae (Namikoshi et al. 2006; Tian et al. 2016). Phthalate esters have been isolated from extracts of marine organisms that cannot be recognized as biological metabolites because phthalates tend to be recognized as artificial plasticizers (Kamio et al. 2021b). These phthalates could be biological molecules, that is natural product. If so, phthalate esters can be natural phagostimulants or deterrents for consumers of their producer organisms. However, this does not mean that it is safe for these phthalates reported as natural products to be distributed in the aquatic environment. The toxicity of the compounds leached from plastic debris and well as the influence of these components on chemical communication and chemosensory behavior of organisms should be tested.
Conclusions and perspectives
Chemical communication among marine organisms has been recognized as an important factor that governs inter- and intraspecific interactions. The physiological basis of chemoreception, especially the identification of chemoreceptor proteins and their genes, including at the single-cell level in model species, is being explored, which will improve our understanding of peripheral chemoreception on chemosensory organs. Additionally, the importance of the rheological environment in benthic foraging has been recognized. Chemically identified cues have been applied to control populations of nuisance fish and to improve feeding efficacy in aquaculture. At the present time there are many unidentified chemical cues, including pheromones, and the elucidation of unidentified cues will be helpful for aquaculture and fisheries. The role of molecules recognized as pheromone candidates will be tested in the biological context.
The reproduction of organisms can be controlled by primer and releaser sex pheromones. Artificial food in aquaculture and bait for fisheries can be improved for more species by identifying unknown attractive molecules, leading to the use of these attractants to selectively catch unwanted species to conserve aquatic communities and ecosystem. While there are many reports of chemical communication, in few of these cases have cue molecules been identified. In decapod crustaceans, only a few molecules are reported as pheromones and candidate pheromones. In fish, more molecules have been reported as pheromones. However, clear results have been obtained in some freshwater fish—but not in marine fish—indicating that a high percentage of salts in the starting material of a pheromone purification process makes purification and thus identification difficult. It is also more difficult to design an efficient assay system for marine organisms than for freshwater organisms. The identification of chemical cues requires purification of the active molecules, high-quality chemistry systems to elucidate the purified molecules, and stable and sensitive bioassays to track the active molecules. To accomplish this, the following issues need to be solved:
-
(1)
Salt issue. The purification of pheromones from marine animals is difficult because the high salt content represents problem in various chromatographic methods. Desalting methods, such as electrodialysis, may overcome the problem.
-
(2)
Behavioral bioassay. Many bioassays used in sex pheromone research are behavioral, which requires using individuals that are ready to respond to sex pheromones. This in turn requires a facility to maintain live animals in a healthy condition and collaborations with aquaculturists and/or aquaria, which are both expensive and time consuming and thus major limitations.
-
(3)
Development of new model species, Aquacultured species could be good models because of their high availability. The aquaculture of decapod crustaceans, including those in their reproductive phase, has not been successful until recently. The white leg shrimp L. vannamei, whose genome has been sequenced, and the kuruma shrimp Marsupenaeus japonicas, which is cultured on a commercial scale, are candidate model species if specific behavioral bioassays can be established. The freshwater species Macrobrachium rosenbergii and Neocaridina denticulate may be more suitable as model species because the pheromone samples would be less salty. The freshwater clam Palaemon paucidens, in which the sternal gland is a candidate pheromone gland, is potentially a good model animal for pheromone research in decapod crustaceans. These animals are SFM species; however, they do not have prolonged precopulatory interactions as do SFM crabs.
The mating behavior of these species must be studied to identify the ideal conditions and behavioral criteria for bioassays. The application of any new method must be effective and may include:
-
(1)
Metabolomic approach. For species where there has been a limited use of bioassays, comparative chemistry, metabolomics and biomarker targeting (Kamio 2009; Kaneko et al. 2018) are effective tools to identify candidate molecules to test in the limited bioassays.
-
(2)
Electro-olfactography. Electro-olfactography to detect the peripheral response of the olfactory epithelium has been effective in narrowing down the list of candidate compounds in fish. Further development of electro-olfactography systems will be helpful for identifying chemical cues in crustaceans and other invertebrates.
Defensive molecules of marine animals and bioactive molecules, including marine toxins, are candidates for antifouling and antiparasitic agents. Extracts of the plant Nephrolepis biserrata have antiparasitic activity against the marine leech Zeylanicobdella arugamensis, an economically important parasite that infests cultured groupers (Shah et al. 2020). In addition to the current library of primary and secondary metabolites, more natural products should be identified. Labile natural product molecules that were not found using conventional methods can now be identified using new approaches, including bioinformatics (Wakimoto and Abe 2012; Wakimoto 2017; Berlinck et al. 2019; Hughes 2021).
Removal of stressful odors and predatory, alarm, and disease cues from aquacultural tanks can provide less stressed conditions. Removing the sources of stressful odors and effective biofiltration can remove these stressful dissolved organic matters (DOM). Understanding the effect of pH on the chemosensory behavior of marine organisms and plasticizers leached from ocean plastics will predict potential effects on marine communities and will provide solutions to these ecological stressors.
Thus, understanding and utilizing chemical cues in marine communities is critical for ensuring the future health of marine ecosystems and sustainable aquaculture and fisheries. At the same time, we must consider the negative impacts of releasing chemicals into the environment, even if the chemicals are natural products (Söffker and Tyler 2012): the release of a bioactive compound in a field has the potential to be harmful to organisms living in that field and to humans living around that field—even if that compound is not acute toxic to the organisms. Although the effects of pheromones are highly species specific and may not affect the activities of other organisms, in laboratory studies, most researchers use only the pheromone of target species at the concentration that induces the expected behavioral or physiological effect, and the effects of the pheromone on other organisms and toxic effects at high concentrations are not tested. When a larger quantity of synthetic pheromones is released into the environment than the natural quantity, it is possible that the synthetic phermones act as toxicants or endocrine disruptors on other animals, including humans. It is therefore important that there be regulations on the use of synthetic bioactive molecules in the environment.
Future research must be interdisciplinary and involve natural products chemists, biochemists, molecular biologists, neuroscientists, physiologists, ecologists, and researchers on aquaculture and fisheries.
Change history
01 December 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12562-021-01571-0
10 March 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12562-022-01590-5
References
Allee WC (1927) Animal aggregations. Q Rev Biol 2:367–398
Allen AS, Seymour AC, Rittschof D (2017) Chemoreception drives plastic consumption in a hard coral. Mar Pollut Bull 124:198–205
Al-Lihaibi SS, Abdel-Lateff A, Alarif WM, Alorfi HS, Nogata Y, Okino T (2019) Environmentally friendly antifouling metabolites from Red Sea organisms. J Chem. J Chem 2019:3278394
Almeida JR, Vasconcelos V (2015) Natural antifouling compounds: effectiveness in preventing invertebrate settlement and adhesion. Biotechnol Adv 33:343–357
Anderson JR, Behringer DC (2013) Spatial dynamics in the social lobster Panulirus argus in response to diseased conspecifics. Mar Ecol Prog Ser 474:191–200
Appelt CW, Sorensen PW (2007) Female goldfish signal spawning readiness by altering when and where they release a urinary pheromone. Anim Behav 74:1329–1338
Arbuckle WJ, Belanger AJ, Corkum LD, Zielinski BS, Li W, Yun SS, Bachynski S, Scott AP (2005) In vitro biosynthesis of novel 5β-reduced steroids by the testis of the round goby, Neogobius melanostomus. Gen Comp Endocrinol 140:1–13
Archdale MV, Añasco CP, Tahara Y (2008) Catches of swimming crabs using fish mince in “teabags” compared to conventional fish baits in collapsible pots. Fish Res 91:291–298
Asai N, Fusetani N, Matsunaga S, Sasaki J (2000) Sex pheromones of the hair crab Erimacrus isenbeckii. Part 1: isolation and structures of novel ceramides. Tetrahedron 56:9895–9899
Asai N, Fusetani N, Matsunaga S (2001) Sex pheromones of the hair crab Erimacrus isenbeckii. II. Synthesis of ceramides. J Nat Prod 64:1210–1215
Ashur MM, Johnston NK, Dixson DL (2017) Impacts of ocean acidification on sensory function in marine organisms. Integr Comp Biol 57:63–80
Atema J (1971) Structures and functions of the sense of taste in the catfish (Ictalurus natalis). Brain Behav Evol 4:273–294
Bailey RJ, Birkett MA, Ingvarsdóttir A, Mordue AJ, Mordue W, O’Shea B, Pickett JA, Wadhams LJ (2006) The role of semiochemicals in host location and non-host avoidance by salmon louse (Lepeophtheirus salmonis) copepodids. Can J Fish Aquat Sci 63:448–456
Bamber S, Naylor E (1997) Sites of release of putative sex pheromone and sexual behaviour in female Carcinus maenas (Crustacea: Decapoda). Estuar Coast Shelf Sci 44:195–202
Barata EN, Hubbard PC, Almeida OG, Miranda A, Canário AVM (2007) Male urine signals social rank in the Mozambique tilapia (Oreochromis mossambicus). BMC Biol 5:54
Barata EN, Fine JM, Hubbard PC, Almeida OG, Frade P, Sorensen PW, Canário AVM (2008) A sterol-like odorant in the urine of Mozambique tilapia males likely signals social dominance to females. J Chem Ecol 34:438–449
Barbato JC, Daniel PC (1997) Chemosensory activation of an antennular grooming behavior in the spiny lobster, Panulirus argus, is tuned narrowly to l-glutamate. Biol Bull 193:107–115
Barki A, Jones C, Karplus I (2010) Chemical communication and aquaculture of decapod crustaceans: needs, problems, and possible solutions. In: Breithaupt T, Thiel M (eds) Chemical communication in crustaceans. Springer, New York, pp 485–506
Batista-Pereira LG, Stein K, de Paula AF, Moreira JA, Cruz I, Figueiredo MdLC, Perri J, Corrêa AG (2006) Isolation, identification, synthesis, and field evaluation of the sex pheromone of the Brazilian population of Spodoptera frugiperda. J Chem Ecol 32:1085–1099
Bauer R (1977) Antifouling adaptations of marine shrimp (Crustacea: Decapoda: Caridea): functional morphology and adaptive significance of antennular preening by the third maxillipeds. Mar Biol 40:261–276
Bauer RT (1981) Grooming behavior and morphology in the decapod Crustacea. J Crust Biol 1:153–173
Behringer DC, Butler MJ, Shields JD (2006) Ecology: avoidance of disease by social lobsters. Nature 441:421
Belanger AJB, Arbuckle WJ, Corkum LD, Gammon DB, Li W, Scott AP, Zielinski BS (2004) Behavioural and electrophysiological responses by reproductive female Neogobius melanostomus to odours released by conspecific males. J Fish Biol 65:933–946
Belanger RM, Pachkowski MD, Stacey NE (2010) Methyltestosterone-induced changes in electro-olfactogram responses and courtship behaviors of cyprinids. Chem Senses 35:65–74
Berlinck RGS, Monteiro AF, Bertonha AF, Bernardi DI, Gubiani JR, Slivinski J, Michaliski LF, Tonon LAC, Venancio VA, Freire VF (2019) Approaches for the isolation and identification of hydrophilic, light-sensitive, volatile and minor natural products. Nat Prod Rep 36:981–1004
Bett NN, Hinch SG (2015) Attraction of migrating adult sockeye salmon to conspecifics in the absence of natal chemical cues. Behav Ecol 26:1180–1187
Bett NN, Hinch SG (2016) Olfactory navigation during spawning migrations: a review and introduction of the hierarchical navigation hypothesis. Biol Rev 91:728–759
Bird DJ, Hamid I, Fox-Rosales L, Van Valkenburgh B (2020) Olfaction at depth: cribriform plate size declines with dive depth and duration in aquatic arctoid carnivorans. Ecol Evol 10:6929–6953
Bjerselius R, Olsén KH, Zheng W (1995) Behavioural and endocrinological responses of mature male goldfish to the sex pheromone 17α, 20β-dihydroxy-4 pregnen-3-one in the water. J Exp Biol 198:747–754
Bjerselius R, Li W, Teeter JH, Seelye JG, Johnsen PB, Maniak PJ, Grant GC, Polkinghorne CN, Sorensen PW (2000) Direct behavioral evidence that unique bile acids released by larval sea lamprey (Petromyzon marinus) function as a migratory pheromone. Can J Fish Aquat Sci 57:557–569
Boone EJ, Boettcher AA, Sherman TD, O’Brien JJ (2003) Characterization of settlement cues used by the rhizocephalan barnacle Loxothylacus texanus. Mar Ecol Prog Ser 252:187–197
Brant CO, Chung-Davidson YW, Li K, Scott AM, Li W (2013) Biosynthesis and release of pheromonal bile salts in mature male sea lamprey. BMC Biochem 14:30
Brant CO, Johnson NS, Li K, Buchinger TJ, Li W (2016a) Female sea lamprey shift orientation toward a conspecific chemical cue to escape a sensory trap. Behav Ecol 27:810–819
Brant CO, Huertas M, Li K, Li W (2016b) Mixtures of two bile alcohol sulfates function as a proximity pheromone in sea lamprey. PLOS ONE 11:e0149508
Broach JS, Phelps RP (2011) Response of male blue catfish, Ictalurus furcatus, and male channel catfish, Ictalurus punctatus, to female channel catfish given pheromonal steroids or prostaglandin. J World Aquac Soc 42:376–387
Brown GE, Smith RJF (1997) Conspecific skin extracts elicit antipredator responses in juvenile rainbow trout (Oncorhynchus mykiss). Can J Zool 75:1916–1922
Brown GE, Smith RJF (1998) Acquired predator recognition in juvenile rainbow trout (Oncorhynchus mykiss): conditioning hatchery-reared fish to recognize chemical cues of a predator. Can J Fish Aquat Sci 55:611–617
Brown GE, Adrian JJC, Patton T, Chivers DP (2001) Fathead minnows learn to recognize predator odour when exposed to concentrations of artificial alarm pheromone below their behavioural-response threshold. Can J Zool 79:2239–2245
Brown GE, Adrian JC, Naderi NT, Harvey MC, Kelly JM (2003) Nitrogen oxides elicit antipredator responses in juvenile channel catfish, but not in convict cichlids or rainbow trout: conservation of the ostariophysan alarm pheromone. J Chem Ecol 29:1781–1796
Brown ER, Cepeda MR, Mascuch SJ, Poulson-Ellestad KL, Kubanek J (2019) Chemical ecology of the marine plankton. Nat Prod Rep 36:1093–1116
Bryan MB, Chung-Davidson YW, Ren JF, Bowman S, Scott AP, Huertas M, Connolly MP, Li W (2015) Evidence that progestins play an important role in spermiation and pheromone production in male sea lamprey (Petromyzon marinus). Gen Comp Endocrinol 212:17–27
Buchinger TJ, Wang H, Li W, Johnson NS (2013) Evidence for a receiver bias underlying female preference for a male mating pheromone in sea lamprey. Proc Roy Soc B 280:20131966
Buchinger TJ, Siefkes MJ, Zielinski BS, Brant CO, Li W (2015) Chemical cues and pheromones in the sea lamprey (Petromyzon marinus). Front Zool 12:32
Buchinger TJ, Bussy U, Buchinger EG, Fissette SD, Li W, Johnson NS (2017a) Increased pheromone signaling by small male sea lamprey has distinct effects on female mate search and courtship. Behav Ecol Sociobiol 71:1–8
Buchinger TJ, Li K, Huertas M, Baker CF, Jia L, Hayes MC, Li W, Johnson NS (2017b) Evidence for partial overlap of male olfactory cues in lampreys. J Exp Biol 220:497–506
Buchinger TJ, Bussy U, Li K, Jia L, Baker CF, Buchinger EG, Zhe Z, Johnson NS, Li W (2019) Intra- and interspecific variation in production of bile acids that act as sex pheromones in lampreys. Physiol Biochem Zool 92:463–472
Buchinger TJ, Scott AM, Fissette SD, Brant CO, Huertas M, Li K, Johnson NS, Li W (2020) A pheromone antagonist liberates female sea lamprey from a sensory trap to enable reliable communication. Proc Natl Acad Sci USA 117:7284–7289
Burns AC, Sorensen PW, Hoye TR (2011) Synthesis and olfactory activity of unnatural, sulfated 5β-bile acid derivatives in the sea lamprey (Petromyzon marinus). Steroids 76:291–300
Bushmann PJ, Atema J (1996) Nephropore rosette glands of the lobster Homarus americanus: possible sources of urine pheromones. J Crust Biol 16:221–231
Butenandt A, Beckmann R, Hecker E (1961) About the sexual attractant of the silk moth, 1. The biological test and the isolation of the pure sexual attractant bombykol. Biol Chem 324:71–83 (in German)
Butler CB, Gutzler BC, Matthews TR (2018a) Sublethal and lethal effects of confinement of Caribbean spiny lobsters, Panulirus argus, in ghost traps. Bull Mar Sci 94:1153–1169
Butler CB, Butler J, Matthews TR (2018b) Starvation reduces attractiveness of live bait lobsters and trap catch in the Caribbean spiny lobster (Panulirus argus) fishery in Florida. Bull Mar Sci 94:1171–1184
Cameron JN, Batterton CV (1978) Antennal gland function in the freshwater blue crab, Callinectes sapidus: water, electrolyte, acid-base and ammonia excretion. J Comp Physiol 123:143–148
Caprio J, Brand JG, Teeter JH, Valentincic T, Kalinoski DL, Kohbara J, Kumazawa T, Wegert S (1993) The taste system of the channel catfish: from biophysics to behavior. Trends Neurosci 16:192–197
Caprio J, Shimohara M, Marui T, Harada S, Kiyohara S (2014) Marine teleost locates live prey through pH sensing. Science 344:1154–1156
Cardwell JR, Dulka JG, Stacey NE (1991) Species-specificity of olfactory responsiveness to potential sex pheromones. In: Scott A, Sumpter J, Kime D, Rolfe M (eds) Proceedings of the Fourth International Symposium on the Reproductive Physiology of Fish. University of Sheffield, Sheffield, p. 201
Cardwell JR, Stacey NE, Tan ESP, McAdam DSO, Lang SLC (1995) Androgen increases olfactory receptor response to a vertebrate sex pheromone. J Comp Physiol A 176:55–61
Carolsfeld J, Tester M, Kreiberg H, Sherwood NM (1997) Pheromone-induced spawning of pacific herring I. Behavioral characterization. Horm Behav 31:256–268
Carr WE (1988) The molecular nature of chemical stimuli in the aquatic environment. In: Atema J, Fay RR, Popper AN, Tavolga WN (eds) Sensory biology of aquatic animals. Springer, New York, pp 3–27
Carr WES, Derby CD (1986) Chemically stimulated feeding behavior in marine animals. J Chem Ecol 12:989–1011
Carr WES, Netherton IJC, Gleeson RA, Derby CD (1996) Stimulants of feeding behavior in fish: analyses of tissues of diverse marine organisms. Biol Bull 190:149–160
Chang ES, Mykles DL (2011) Regulation of crustacean molting: a review and our perspectives. Gen Comp Endocrinol 172:323–330
Chapman J, Hellio C, Sullivan T, Brown R, Russell S, Kiterringham E, Le Nor L, Regan F (2014) Bioinspired synthetic macroalgae: examples from nature for antifouling applications. Int Biodeterior Biodegrad 86:6–13
Chiba S, Yoshino K, Kanaiwa M, Kawajiri T, Goshima S (2013) Maladaptive sex ratio adjustment by a sex-changing shrimp in selective-fishing environments. J Anim Ecol 82:632–641
Chung-Davidson YW, Rees CB, Bryan MB, Li W (2008) Neurogenic and neuroendocrine effects of goldfish pheromones. J Neurosci 28:14492–14499
Chung-Davidson YW, Wang H, Bryan MB, Wu H, Johnson NS, Li W (2013a) An anti-steroidogenic inhibitory primer pheromone in male sea lamprey (Petromyzon marinus). Gen Comp Endocrinol 189:24–31
Chung-Davidson YW, Wang HY, Siefkes MJ, Bryan MB, Wu H, Johnson NS, Li W (2013b) Pheromonal bile acid 3-ketopetromyzonol sulfate primes the neuroendocrine system in sea lamprey. BMC Neurosci 14:11
Chung-Davidson YW, Bussy U, Fissette SD, Li W (2020) Sex-dependent pheromonal effects on steroid hormone levels in sea lampreys (Petromyzon marinus). Gen Comp Endocrinol 299:113608
Colombo L, Marconato A, Belvedere PC, Friso C (1980) Endocrinology of teleost reproduction: a testicular steroid pheromone in the black goby, Gobius jozo L. Boll Zool 47:355–364
Corkum LD, Meunier B, Moscicki M, Zielinski BS, Scott AP (2008) Behavioural responses of female round gobies (Neogobius melanostomus) to putative steroidal pheromones. Behaviour 145:1347–1365
Cummins SF, Nichols AE, Amare A, Hummon AB, Sweedler JV, Nagle GT (2004) Characterization of Aplysia enticin and temptin, two novel water-borne protein pheromones that act in concert with attractin to stimulate mate attraction. J Biol Chem 279:25614–25622
Dana E, Sara C, Hanh N-V, Charles DD (2020) Chemosensory basis of feeding behavior in Pacific white shrimp, Litopenaeus vannamei. Biol Bull 239:115–131
Davies NB, Krebs JR, West SA (2012) An introduction to behavioural ecology. Wiley, Oxford
Defraipont M, Sorensen PW (1993) Exposure to the pheromone 17α, 20β-dihydroxy-4-pregnen-3-one enhances the behavioural spawning success, sperm production and sperm motility of male goldfish. Anim Behav 46:245–256
Del Monaco C, Hay ME, Gartrell P, Mumby PJ, Diaz-Pulido G (2017) Effects of ocean acidification on the potency of macroalgal allelopathy to a common coral. Sci Rep 7:41053
Derby CD (2014) Cephalopod ink: production, chemistry, functions and applications. Mar Drugs 12:2700–2730
Derby CD (2020) Chemoreception in aquatic invertebrates. In: Fritzsch B (ed) The senses: a comprehensive reference, 2nd edn. Elsevier, Oxford, pp 65–84
Derby CD (2021) The crustacean antennule: a complex organ adapted for lifelong function in diverse environments and lifestyles. Biol Bull 240:67–81
Derby CD, Zimmer RK (2012) Neuroecology of predator-prey interactions. In: Brönmark C, Hansson L-A (eds) Chemical ecology in aquatic systems. Oxford University Press, New York, pp 158–171
Derby CD, Kicklighter CE, Johnson PM, Zhang X (2007) Chemical composition of inks of diverse marine molluscs suggests convergent chemical defenses. J Chem Ecol 33:1105–1113
Derby CD, Kozma MT, Senatore A, Schmidt M (2016a) Molecular mechanisms of reception and perireception in crustacean chemoreception: a comparative review. Chem Senses 41:381–398
Derby CD, Elsayed FH, Williams SA, González C, Choe M, Bharadwaj AS, Chamberlain GW (2016b) Krill meal enhances performance of feed pellets through concentration-dependent prolongation of consumption by Pacific white shrimp, Litopenaeus vannamei. Aquaculture 458:13–20
Derby C, Bharadwaj A, Chamberlain G (2018) Development of a sustainable natural chemostimulant for shrimp feed. AquaFeed 10:39–43
Di Rocco RT, Imre I, Johnson NS, Brown GE (2015) Behavioural response of adult sea lamprey (Petromyzon marinus) to predator and conspecific alarm cues: evidence of additive effects. Hydrobiologia 767:279–287
Diana Z, Sawickij N, Rivera NA Jr, Hsu-Kim H, Rittschof D (2020) Plastic pellets trigger feeding responses in sea anemones. Aquat Toxicol 222:105447
Dillman AR, Guillermin ML, Lee JH, Kim B, Sternberg PW, Hallem EA (2012) Olfaction shapes host–parasite interactions in parasitic nematodes. Proc Natl Acad Sci USA 109:E2324–E2333
Dong S, Ohara K, Taniguchi N (1997) Introduction of sperm of common carp Cyprinus carpio into eggs of ginbuna Carassius langsdorfii by heat shock treatment and its confirmation by DNA markers. Nippon Suisan Gakkaishi 63:201–206
Dreanno C, Kirby RR, Clare AS (2007) Involvement of the barnacle settlement-inducing protein complex (SIPC) in species recognition at settlement. J Exp Mar Biol Ecol 351:276–282
Dulka JG, Stacey NE, Sorensen PW, Van Der Kraak GJ (1987) A steroid sex pheromone synchronizes male–female spawning readiness in goldfish. Nature 325:251–253
Dunn PH, Young CM (2014) Larval settlement of the nemertean egg predator Carcinonemertes errans on the Dungeness crab, Metacarcinus magister. Invertebr Biol 133:201–212
Endo H, Wu H (2019) Biosensors for the assessment of fish health: a review. Fish Sci 85:641–654
Ferner MC, Weissburg MJ (2005) Slow-moving predatory gastropods track prey odors in fast and turbulent flow. J Exp Biol 208:809–819
Ferner MC, Smee DL, Chang YP (2005) Cannibalistic crabs respond to the scent of injured conspecifics: danger or dinner? Mar Ecol Prog Ser 300:193–200
Ferner MC, Smee DL, Weissburg MJ (2009) Habitat complexity alters lethal and non-lethal olfactory interactions between predators and prey. Mar Ecol Prog Ser 374:13–22
Ferrier GA, Kim SJ, Kaddis CS, Loo JA, Ann Zimmer C, Zimmer RK (2016) MULTIFUNCin: A multifunctional protein cue induces habitat selection by, and predation on, barnacles. Integr Comp Biol 56:901–913
Fine JM, Sorensen PW (2008) Isolation and biological activity of the multi-component sea lamprey migratory pheromone. J Chem Ecol 34:1259–1267
Frade P, Hubbard PC, Barata EN, Canário AVM (2002) Olfactory sensitivity of the Mozambique tilapia to conspecific odours. J Fish Biol 61:1239–1254
Fraser EJ, Stacey N (2002) Isolation increases milt production in goldfish. J Exp Zool 293:511–524
Fredricks K, Johnson N, Hubert T, Siefkes M (2020) Registration and application of sea lamprey pheromones for sea lamprey control in the United States and Canada. J Great Lakes Res. https://doi.org/10.1016/j.jglr.2020.07.017
Friedrich RW, Korsching SI (1998) Chemotopic, combinatorial, and noncombinatorial odorant representations in the olfactory bulb revealed using a voltage-sensitive axon tracer. J Neurosci 18:9977–9988
Frisch KV (1938) Zur psychologie des fisch-schwarmes [Psychology of preference in fish]. Naturwissenschaften 26:601–606 (in German)
Frisch KV (1942) About a frightening substance of the fish skin and its biological significance. Z Vergl Physiol 29:46–145 (in German)
Fujimoto Y, Yambe H, Takahashi K, Sato S (2020) Bile from reproductively mature male largemouth bass Micropterus salmoides attracts conspecific females and offers a practical application to control populations. Manag Biol Invasion 11:415–427
Fusetani N (2004) Biofouling and antifouling. Nat Prod Rep 21:94–104
Ghosal R, Eichmiller JJ, Witthuhn BA, Sorensen PW (2018) Attracting common carp to a bait site with food reveals strong positive relationships between fish density, feeding activity, environmental DNA, and sex pheromone release that could be used in invasive fish management. Ecol Evol 8:6714–6727
Giaquinto PC, Hara TJ (2008) Discrimination of bile acids by the rainbow trout olfactory system: evidence as potential pheromone. Biol Res 41:33–42
Gleeson RA (1980) Pheromone communication in the reproductive behavior of the blue crab, Callinectes sapidus. Mar Behav Physiol 7:119–134
Gleeson R (1991) Intrinsic factors mediating pheromone communication in the blue crab, Callinectes sapidus. In: Bauer RT, Martin JW (eds) Crustacean sexual biology. Columbia University Press, New York, pp 17–32
Gleeson RA, Adams MA, Smith AB (1984) Characterization of a sex pheromone in the blue crab, Callinectes sapidus: crustecdysone studies. J Chem Ecol 10:913–921
Gleeson RA, McDowell LM, Aldrich HC (1996) Structure of the aesthetasc (olfactory) sensilla of the blue crab, Callinectes sapidus: transformations as a function of salinity. Cell Tissue Res 284:279–288
Greer PL, Bear DM, Lassance J-M, Bloom ML, Tsukahara T, Pashkovski SL, Masuda FK, Nowlan AC, Kirchner R, Hoekstra HE, Datta SR (2016) A family of non-GPCR chemosensors defines an alternative logic for mammalian olfaction. Cell 165:1734–1748
Haas W (2003) Parasitic worms: strategies of host finding, recognition and invasion. Zoology 106:349–364
Hara TJ (1994) The diversity of chemical stimulation in fish olfaction and gustation. Rev Fish Biol Fisheries 4:1–35
Hardege J, Jennings A, Hayden D, Müller C, Pascoe D, Bentley M, Clare A (2002) Novel behavioural assay and partial purification of a female-derived sex pheromone in Carcinus maenas. Mar Ecol Prog Ser 244:179–189
Hardege J, Bartels-Hardege H, Fletcher N, Terschak J, Harley M, Smith M, Davidson L, Hayden D, Müller CT, Lorch M (2011) Identification of a female sex pheromone in Carcinus maenas. Mar Ecol Prog Ser 436:177–189
Harder T, Tebben J, Möller M, Schupp PJ (2018) Chemical ecology of marine invertebrate larval settlement. In: Melany PP, Mikel AB (eds) Chemical ecology. CRC Press, Boca Raton, pp 329–355
Hartnoll R (1969) Mating in the Brachyura. Crustaceana 16:161–181
Hay ME (2009) Marine chemical ecology: Chemical signals and cues structure marine populations, communities, and ecosystems. Annu Rev Mar Sci 1:193–212
Hay ME, Fenical W (1988) Marine plant–herbivoe interactions: the ecology of chemical defense. Annu Rev Ecol Syst 19:111–145
Hay ME, Kubanek J (2002) Community and ecosystem level consequences of chemical cues in the plankton. J Chem Ecol 28:2001–2016
Hayashihara N, Kamio M (2016) Chemical defenses in the skin and the ink of sea hares Aplysia juliana and Aplysia gigantea. Jpn J Benthol 71:11–16 (in Japanese with English abstract)
Hellstrom G, Prestegaard T, Dannewitz J, Olsén KH (2013) Sperm from pheromone primed brown trout (Salmo trutta L.) produce more larvae. Fish Physiol Biochem 39:471–478
Hermabessiere L, Dehaut A, Paul-Pont I, Lacroix C, Jezequel R, Soudant P, Duflos G (2017) Occurrence and effects of plastic additives on marine environments and organisms: a review. Chemosphere 182:781–793
Heuschele J, Candolin U (2007) An increase in pH boosts olfactory communication in sticklebacks. Biol Lett 3:411–413
Hevonoja T, Pentikäinen MO, Hyvönen MT, Kovanen PT, Ala-Korpela M (2000) Structure of low density lipoprotein (LDL) particles: Basis for understanding molecular changes in modified LDL. Biochim Biophys Acta 1488:189–210
Hill JM, Weissburg MJ (2014) Crabs interpret the threat of predator body size and biomass via cue concentration and diet. Anim Behav 92:117–123
Hino H, Iwai T, Yamashita M, Ueda H (2007) Identification of an olfactory imprinting-related gene in the lacustrine sockeye salmon, Oncorhynchus nerka. Aquaculture 273:200–208
Horner AJ, Nickles SP, Weissburg MJ, Derby CD (2006) Source and specificity of chemical cues mediating shelter preference of Caribbean spiny lobsters (Panulirus argus). Biol Bull 211:128–139
Horner AJ, Weissburg MJ, Derby CD (2008) The olfactory pathway mediates sheltering behavior of Caribbean spiny lobsters, Panulirus argus, to conspecific urine signals. J Comp Physiol A 194:243–253
Hoysak D, Stacey N (2008) Large and persistent effect of a female steroid pheromone on ejaculate size in goldfish Carassius auratus. J Fish Biol 73:1573–1584
Huertas M, Scott AP, Hubbard PC, Canário AV, Cerdà J (2006) Sexually mature European eels (Anguilla anguilla L.) stimulate gonadal development of neighbouring males: possible involvement of chemical communication. Gen Comp Endocrinol 147:304–313
Huertas M, Almeida OG, Canário AVM, Hubbard PC (2014) Tilapia male urinary pheromone stimulates female reproductive axis. Gen Comp Endocrinol 196:106–111
Hughes CC (2021) Chemical labeling strategies for small molecule natural product detection and isolation. Nat Prod Rep 38:1684–1705
Hungria DB, dos Santos Tavares CP, Pereira LÂ, da de Assis Teixeira Silva U, Ostrensky A (2017) Global status of production and commercialization of soft-shell crabs. Aquac Int 25:2213–2226
Hunt JH (2000) Status of the fishery for Panulirus argus in Florida. In: Phillips BF, Kittaka J (eds) Spiny lobsters: fisheries and culture. Fishing News Books, Oxford, pp 189–199
Hunt JH, Lyons WG, Kennedy FS Jr (1986) Effects of exposure and confinement on spiny lobsters, Panulirus argus, used as attractants in the Florida trap fishery. Fish Bull 84:69–76
Ianora A, Bentley MG, Caldwell GS, Casotti R, Cembella AD, Engstrom-Ost J, Halsband C, Sonnenschein E, Legrand C, Llewellyn CA, Paldaviciene A, Pilkaityte R, Pohnert G, Razinkovas A, Romano G, Tillmann U, Vaiciute D (2011) The relevance of marine chemical ecology to plankton and ecosystem function: an emerging field. Mar Drugs 9:1625–1648
Ingvarsdóttir A, Birkett MA, Duce I, Genna RL, Mordue W, Pickett JA, Wadhams LJ, Mordue AJ (2002) Semiochemical strategies for sea louse control: host location cues. Pest Manag Sci 58:537–545
Ishizawa S, Yamamoto Y, Denboh T, Ueda H (2010) Release of dissolved free amino acids from biofilms in stream water. Fish Sci 76:669–676
Isobe A, Iwasaki S, Uchida K, Tokai T (2019) Abundance of non-conservative microplastics in the upper ocean from 1957 to 2066. Nat Commun 10:417
Jager JC (1970) A quantitative study of a chemoresponse to sugars in Lymnaea stagnalis (L.). Neth J Zool 21:1–59
Jivoff P, Hines AH, Quackenbush LS (2007) Reproduction biology and embryonic development. In: Kennedy VS, Cronin LE (eds) The Blue Crab Callinectes sapidus. Maryland Sea Grant College, College Park, pp 255–298
Johansen PH (1985) Female pheromone and the behaviour of male guppies (Poecilia reticulata) in a temperature gradient. Can J Zool 63:1211–1213
Johnson PM, Kicklighter CE, Schmidt M, Kamio M, Yang H, Elkin D, Michel WC, Tai PC, Derby CD (2006) Packaging of chemicals in the defensive secretory glands of the sea hare Aplysia californica. J Exp Biol 209:78–88
Johnson NS, Yun SS, Thompson HT, Brant CO, Li W (2009) A synthesized pheromone induces upstream movement in female sea lamprey and summons them into traps. Proc Natl Acad Sci USA 106:1021–1026
Johnson NS, Yun SS, Buchinger TJ, Li W (2012) Multiple functions of a multi-component mating pheromone in sea lamprey Petromyzon marinus. J Fish Biol 80:538–554
Johnson NS, Siefkes MJ, Wagner CM, Dawson H, Wang H, Steeves T, Twohey M, Li W (2013) A synthesized mating pheromone component increases adult sea lamprey (Petromyzon marinus) trap capture in management scenarios. Can J Fish Aquat Sci 70:1101–1108
Johnson NS, Yun SS, Li W (2014) Investigations of novel unsaturated bile salts of male sea lamprey as potential chemical cues. J Chem Ecol 40:1152–1160
Johnson NS, Siefkes MJ, Wagner CM, Bravener G, Steeves T, Twohey M, Li W (2015) Factors influencing capture of invasive sea lamprey in traps baited with a synthesized sex pheromone component. J Chem Ecol 41:913–923
Johnson NS, Lewandoski SA, Alger BJ, O’Connor L, Bravener G, Hrodey P, Huerta B, Barber J, Li W, Wagner CM, Siefkes MJ (2020) Behavioral responses of sea lamprey to varying application rates of a synthesized pheromone in diverse trapping scenarios. J Chem Ecol 46:233–249
Kamiguchi Y (1972) A histological study of the “sternal gland” in the female freshwater prawn, Palaemon paucidens, a possible site of origin of the sex pheromone. J Fac Sci Hokkaido Univ Ser 6(18):356–365
Kamio M (2009) Toward identifying sex pheromones in blue crabs. Ann NY Acad Sci 1170:456–461
Kamio M, Derby CD (2010) Approaches to a molecular identification of sex pheromones in blue crabs. In: Breithaupt T, Thiel M (eds) Chemical communication in crustaceans. Springer, New York, pp 393–412
Kamio M, Derby CD (2017) Finding food: how marine invertebrates use chemical cues to track and select food. Nat Prod Rep 34:514–528
Kamio M, Matsunaga S, Fusetani N (2000) Studies on sex pheromones of the helmet crab, Telmessus cheiragonus 1. An assay based on precopulatory mate-guarding. Zool Sci 17:731–733
Kamio M, Matsunaga S, Fusetani N (2002) Copulation pheromone in the crab Telmessus cheiragonus (Brachyura : Decapoda). Mar Ecol Prog Ser 234:183–190
Kamio M, Matsunaga S, Fusetani N (2003) Observation on the mating behaviour of the helmet crab Telmessus cheiragonus (Brachyura : Cheiragonidae). J Mar Biol Assoc UK 83:1007–1013
Kamio M, Araki M, Nagayama T, Matsunaga S, Fusetani N (2005) Behavioral and electrophysiological experiments suggest that the antennular outer flagellum is the site of pheromone reception in the male helmet crab Telmessus cheiragonus. Biol Bull 208:12–19
Kamio M, Reidenbach MA, Derby CD (2008) To paddle or not: context dependent courtship display by male blue crabs, Callinectes sapidus. J Exp Biol 211:1243–1248
Kamio M, Ko KC, Zheng S, Wang B, Collins SL, Gadda G, Tai PC, Derby CD (2009) The chemistry of escapin: identification and quantification of the components in the complex mixture generated by an L-amino acid oxidase in the defensive secretion of the sea snail Aplysia californica. Chem Eur J 15:1597–1603
Kamio M, Grimes TV, Hutchins MH, van Dam R, Derby CD (2010a) The purple pigment aplysioviolin in sea hare ink deters predatory blue crabs through their chemical senses. Anim Behav 80:89–100
Kamio M, Nguyen L, Yaldiz S, Derby CD (2010b) How to produce a chemical defense: structural elucidation and anatomical distribution of aplysioviolin and phycoerythrobilin in the sea hare Aplysia californica. Chem Biodivers 7:1183–1197
Kamio M, Kicklighter CE, Nguyen L, Germann MW, Derby CD (2011) Isolation and structural elucidation of novel mycosporine-like amino acids as alarm cues in the defensive ink secretion of the sea hare Aplysia californica. Helv Chim Acta 94:1012–1018
Kamio M, Schmidt M, Germann MW, Kubanek J, Derby CD (2014) The smell of moulting: N-acetylglucosamino-1,5-lactone is a premoult biomarker and candidate component of the courtship pheromone in the urine of the blue crab, Callinectes sapidus. J Exp Biol 217:1286–1296
Kamio M, Furukawa D, Wakabayashi K, Hiei K, Yano H, Sato H, Yoshie-Stark Y, Akiba T, Tanaka Y (2015) Grooming behavior by elongated third maxillipeds of phyllosoma larvae of the smooth fan lobster riding on jellyfishes. J Exp Mar Biol Ecol 463:115–124
Kamio M, Koyama M, Hayashihara N, Hiei K, Uchida H, Watanabe R, Suzuki T, Nagai H (2016a) Sequestration of dimethylsulfoniopropionate (DMSP) and acrylate from the green alga Ulva spp. by the sea hare Aplysia juliana. J Chem Ecol 42:452–460
Kamio M, Wakabayashi K, Nagai H, Tanaka Y (2016b) Phyllosomas of smooth fan lobsters (Ibacus novemdentatus) encase jellyfish cnidae in peritrophic membranes in their feces. Plankton Benthos Res 11:100–104
Kamio M, Nagakura Y, Yano H (2017) The molting biomarker molecule exists as 2-acetamido-2-deoxy-gluconic acid in urine of blue crabs and helmet crabs. Chem Biodivers 14:e1700063
Kamio M, Osada H, Yano H (2021a) Morphological and behavioral indicators of reproductive premolt females of the helmet crab Telmessus cheiragonus. Fish Sci 87:331–337
Kamio M, Jiang W, Osada H, Fukuoka M, Uchida H, Watanabe R, Suzuki T, Nagai H (2021b) Isolation and structure elucidation of a novel symmetrical macrocyclic phthalate hexaester. Symmetry 13:361
Kamiya H, Sakai R, Jimbo M (2006) Bioactive molecules from sea hares. In: Guido C, Margherita G (eds) Molluscs, progress in molecular and subcellular biology. Springer, Berlin, pp 215–239
Kamyab E, Rohde S, Kellermann MY, Schupp PJ (2020) Chemical defense mechanisms and ecological implications of Indo-Pacific holothurians. Molecules 25:4808
Kaneko G, Ushio H, Ji H (2018) Application of magnetic resonance technologies in aquatic biology and seafood science. Fish Sci 85:1–17
Katare YK, Scott AP, Laframboise AJ, Li W, Alyasha’e Z, Caputo CB, Loeb SJ, Zielinski B (2011) Release of free and conjugated forms of the putative pheromonal steroid 11-oxo-etiocholanolone by reproductively mature male round goby (Neogobius melanostomus Pallas, 1814). Biol Reprod 84:288–298
Katsel PL, Dmitrieva TM, Valeyev RB, Kozlov YP (1992) Sex pheromones of male yellowfin baikal sculpin (Cottocomephorus grewingki): isolation and chemical studies. J Chem Ecol 18:2003–2010
Kawabata K (1993) Induction of sexual behavior in male fish (Rhodeus ocellatus ocellatus) by amino acids. Amino Acids 5:323–327
Kawamura G, Matsuoka T, Tajiri T, Nishida M, Hayashi M (1995) Effectiveness of a sugarcane-fish combination as bait in trapping swimming crabs. Fish Res 22:155–160
Keller-Costa T, Lopes OS, Almeida O, Hubbard PC, Iacovella A, Lima M, Barata EN, Canário AVM (2012) Muscular hypertrophy of urinary bladders in dominant tilapia facilitates the control of aggression through urinary signals. Behaviour 149:953–975
Keller-Costa T, Hubbard PC, Paetz C, Nakamura Y, da Silva JP, Rato A, Barata EN, Schneider B, Canário AVM (2014a) Identity of a tilapia pheromone released by dominant males that primes females for reproduction. Curr Biol 24:2130–2135
Keller-Costa T, Canário AVM, Hubbard PC (2014b) Olfactory sensitivity to steroid glucuronates in Mozambique tilapia suggests two distinct and specific receptors for pheromone detection. J Exp Biol 217:4203–4212
Keller-Costa T, Saraiva JL, Hubbard PC, Barata EN, Canário AVM (2016) A multi-component pheromone in the urine of dominant male tilapia (Oreochromis mossambicus) reduces aggression in rivals. J Chem Ecol 42:173–182
Keverne EB (1999) The vomeronasal organ. Science 286:716–720
Kicklighter CE, Shabani S, Johnson PM, Derby CD (2005) Sea hares use novel antipredatory chemical defenses. Curr Biol 15:549–554
Kicklighter CE, Germann M, Kamio M, Derby CD (2007) Molecular identification of alarm cues in the defensive secretions of the sea hare Aplysia californica. Anim Behav 74:1481–1492
Kicklighter CE, Kamio M, Nguyen L, Germann MW, Derby CD (2011) Mycosporine-like amino acids are multifunctional molecules in sea hares and their marine community. Proc Natl Acad Sci USA 108:11494–11499
Kiesecker JM, Skelly DK, Beard KH, Preisser E (1999) Behavioral reduction of infection risk. Proc Natl Acad Sci USA 96:9165–9168
Kikuyama S, Toyoda F, Yamamoto K, Iwata T, Nakada T, Hasunuma I (2013) Sodefrin and related pheromones. In: Kastin A (ed) Handbook of biologically active peptides. Elsevier Science, San Diego, pp 384–390
Kinoshita N, Nagasato C, Motomura T (2017) Phototaxis and chemotaxis of brown algal swarmers. J Plant Res 130:443–453
Kishida T (2021) Olfaction of aquatic amniotes. Cell Tissue Res 383:353–365
Kitamura S, Ikuta K (2000) Acidification severely suppresses spawning of hime salmon (land-locked sockeye salmon, Oncorhynchus nerka). Aquat Toxicol 51:107–113
Kitamura S, Ogata H, Takashima F (1994a) Olfactory responses of several species of teleost to F-prostaglandins. Comp Biochem Physiol A Mol Integr Physiol A 107:463–467
Kitamura S, Ogata H, Takashima F (1994b) Activities of F-type prostaglandins as releaser sex pheromones in cobitide loach, Misgurnus anguillicaudatus. Comp Biochem Physiol A Mol Integr Physiol 107:161–169
Kittredge JS, Terry M, Takahashi FT (1971) Sex pheromone activity of the molting hormone, crustecdysone, on male crabs. Fish Bull 69:337–343
Kobayashi M, Sorensen PW, Stacey NE (2002) Hormonal and pheromonal control of spawning behavior in the goldfish. Fish Physiol Biochem 26:71–84
Kong CH, Zhang SZ, Li YH, Xia ZC, Yang XF, Meiners SJ, Wang P (2018) Plant neighbor detection and allelochemical response are driven by root-secreted signaling chemicals. Nat Commun 9:386
Koya Y, Fujii R, Yambe H, Tahara D (2015) Hypertrophy and polysaccharide production in the kidney associated with sexual maturation of male small-egged Kajika, Cottus pollux SE. Ichthyol Res 63:260–266
Koya Y, Fujii R, Yambe H, Tahara D (2016) Nesting behavior is associated with increased urinary volume in the urinary bladder during the reproductive period in small-egged Kajika, Cottus pollux SE. Ichthyol Res 63:59–67
Kozma MT, Ngo-Vu H, Wong YY, Shukla NS, Pawar SD, Senatore A, Schmidt M, Derby CD (2020) Comparison of transcriptomes from two chemosensory organs in four decapod crustaceans reveals hundreds of candidate chemoreceptor proteins. PLOS ONE 15:e0230266
Kozma MT, Ngo-Vu H, Rump MT, Bobkov YV, Ache BW, Derby CD (2020b) Single cell transcriptomes reveal expression patterns of chemoreceptor genes in olfactory sensory neurons of the Caribbean spiny lobster Panulirus Argus. BMC Genomics 21:649
Krång A-S, Knaden M, Steck K, Hansson BS (2012) Transition from sea to land: olfactory function and constraints in the terrestrial hermit crab Coenobita clypeatus. Proc Roy Soc B 279:3510–3519
Laberge F, Hara TJ (2001) Neurobiology of fish olfaction: a review. Brain Res Rev 36:46–59
Laberge F, Hara TJ (2003) Behavioral and electrophysiological responses to F-prostaglandins, putative spawning pheromones, in three salmonid fishes. J Fish Biol 62:206–221
Lado WE, Zhang D, Mennigen JA, Zamora JM, Popesku JT, Trudeau VL (2013) Rapid modulation of gene expression profiles in the telencephalon of male goldfish following exposure to waterborne sex pheromones. Gen Comp Endocrinol 192:204–213
Laframboise AJ, Zielinski BS (2011) Responses of round goby (Neogobius melanostomus) olfactory epithelium to steroids released by reproductive males. J Comp Physiol A 197:999–1008
Lambert J, Resink J (1991) Steroid glucuronides as male pheromones in the reproduction of the African catfish Clarias gariepinus—a brief review. J Steroid Biochem Mol Biol 40:549–556
Large SI, Smee DL, Trussell GC (2011) Environmental conditions influence the frequency of prey responses to predation risk. Mar Ecol Prog Ser 422:41–49
Leduc AO, Munday PL, Brown GE, Ferrari MC (2013) Effects of acidification on olfactory-mediated behaviour in freshwater and marine ecosystems: a synthesis. Philos Trans R Soc B 368:20120447
Lefèvre PL, Palin M-F, Murphy BD (2011) Polyamines on the reproductive landscape. Endocr Rev 32:694–712
Levert A, Foulon V, Fauchon M, Tapissier-Bontemps N, Banaigs B, Hellio C (2020) Antifouling activity of meroterpenes isolated from the ascidian Aplidium aff. densum. Mar Biotechnol 23:51-61 I
Levesque HM, Scaffidi D, Polkinghorne CN, Sorensen PW (2011) A multi-component species identifying pheromone in the goldfish. J Chem Ecol 37:219–227
Li W, Sorensen PW (1997) Highly independent olfactory receptor sites for naturally occurring bile acids in the sea lamprey, Petromyzon marinus. J Comp Physiol A 180:429–438
Li W, Scott AP, Siefkes MJ, Yan H, Liu Q, Yun S-S, Gage DA (2002) Bile acid secreted by male sea lamprey that acts as a sex pheromone. Science 296:138–141
Li W, Twohey M, Jones M, Wagner M (2007) Research to guide use of pheromones to control sea lamprey. J Great Lakes Res 33:70–86
Li K, Brant CO, Siefkes MJ, Kruckman HG, Li W (2013) Characterization of a novel bile alcohol sulfate released by sexually mature male sea lamprey (Petromyzon marinus). PLOS ONE 8:e68157
Li K, Scott AM, Riedy JJ, Fissette S, Middleton ZE, Li W (2017) Three novel bile alcohols of mature male sea lamprey (Petromyzon marinus) act as chemical cues for conspecifics. J Chem Ecol 43:543–549
Li K, Brant CO, Huertas M, Hessler EJ, Mezei G, Scott AM, Hoye TR, Li W (2018) Fatty-acid derivative acts as a sea lamprey migratory pheromone. Proc Natl Acad Sci USA 115:8603–8608
Li J, Hubbard PC, Canário AVM (2018) Male zebrafish (Danio rerio) odorants attract females and induce spawning. Aquac Fish 3:139–144
Liley NR, Rouger Y (1990) Plasma levels of gonadotropin and 17α,20β-dihydroxy-4-pregnen-3-one in relation to spawning behaviour of rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Biol 37:699–711
Lim H, Sorensen PW (2011) Polar metabolites synergize the activity of prostaglandin F2α in a species-specific hormonal sex pheromone released by ovulated common carp. J Chem Ecol 37:695–704
Lim H, Sorensen PW (2012) Common carp implanted with prostaglandin F2α release a sex pheromone complex that attracts conspecific males in both the laboratory and field. J Chem Ecol 38:127–134
Liu L-L, Wu C-H, Qian P-Y (2020) Marine natural products as antifouling molecules—a mini-review (2014–2020). Biofouling 36:1210–1226
Lokvam J, Brenes-Arguedas T, Lee JS, Coley PD, Kursar TA (2006) Allelochemic function for a primary metabolite: the case of L-tyrosine hyper-production in Inga umbellifera (Fabaceae). Am J Bot 93:1109–1115
Lozano-Álvarez E, Cid-González LN, Candia-Zulbarán RI, Negrete-Soto F, Barradas-Ortiz C, Briones-Fourzán P (2018) Avoiding disease vs avoiding predation: testing the trade-off in Panulirus argus. Bull Mar Sci 94:657–674
Machnes Z, Avtalion R, Shirak A, Trombka D, Wides R, Fellous M, Don J (2008) Male-specific protein (MSP): a new gene linked to sexual behavior and aggressiveness of tilapia males. Horm Behav 54:442–449
Machon J, Ravaux J, Zbinden M, Lucas P (2016) New electroantennography method on a marine shrimp in water. J Exp Biol 219:3696–3700
Machon J, Lucas P, Ravaux J, Zbinden M (2018) Comparison of chemoreceptive abilities of the hydrothermal shrimp Mirocaris fortunata and the coastal shrimp Palaemon elegans. Chem Senses 43:489–501
Mato Y, Isobe T, Takada H, Kanehiro H, Ohtake C, Kaminuma T (2001) Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ Sci Technol 35:318–324
Matsumura K (1995) Tetrodotoxin as a pheromone. Nature 378:563–564
Matsumura K, Nagano M, Fusetani N (1998) Purification of a larval settlement-inducing protein complex (SIPC) of the barnacle, Balanus amphitrite. J Exp Zool 281:12–20
Matsumura K, Matsunaga S, Fusetani N (2004) Possible involvement of phosphatidylcholine in school recognition in the catfish, Plotosus lineatus. Zool Sci 21:257–264
Matsumura K, Matsunaga S, Fusetani N (2007) Phosphatidylcholine profile-mediated group recognition in catfish. J Exp Biol 210:1992–1999
McLennan DA (2003) The importance of olfactory signals in the gasterosteid mating system: sticklebacks go multimodal. Biol J Linn Soc 80:555–572
McLennan DA (2005) Changes in response to olfactory cues across the ovulatory cycle in brook sticklebacks, Culaea inconstans. Anim Behav 69:181–188
Meckley TD, Wagner CM, Gurarie E, Tierney K (2014) Coastal movements of migrating sea lamprey (Petromyzon marinus) in response to a partial pheromone added to river water: implications for management of invasive populations. Can J Fish Aquat Sci 71:533–544
Miranda A, Almeida OG, Hubbard PC, Barata EN, Canário AVM (2005) Olfactory discrimination of female reproductive status by male tilapia (Oreochromis mossambicus). J Exp Biol 208:2037–2043
Moir F, Weissburg M (2009) Cautious cannibals: behavioral responses of juvenile and adult blue crabs to the odor of injured conspecifics. J Exp Mar Biol Ecol 369:87–92
Moon B, Park YC, McClintock JB, Baker BJ (2000) Structure and bioactivity of erebusinone, a pigment from the Antarctic sponge Isodictya erinacea. Tetrahedron 56:9057–9062
Moore A, Waring CP (1996) Electrophysiological and endocrinological evidence that F-series prostaglandins function as priming pheromones in mature male Atlantic salmon (Salmo salar) parr. J Exp Biol 199:2307–2316
Moore A, Olsén KH, Lower N, Kindahl H (2002) The role of F-series prostaglandins as reproductive priming pheromones in the brown trout. J Fish Biol 60:613–624
Morais S, Derby C (2019) A palatability enhancer that improves the performance of feed pellets in shrimp aquaculture. AquaFeed 11:48–49
Mordue Luntz AJ, Birkett MA (2009) A review of host finding behaviour in the parasitic sea louse, Lepeophtheirus salmonis (Caligidae: Copepoda). J Fish Dis 32:3–13
Murphy CA, Stacey NE (2002) Methyl-testosterone induces male-typical ventilatory behavior in response to putative steroidal pheromones in female round gobies (Neogobius melanostomus). Horm Behav 42:109–115
Murphy CA, Stacey NE, Corkum LD (2001) Putative steroidal pheromones in the round goby, Neogobius melanostomus: olfactory and behavioral responses. J Chem Ecol 27:443–470
Nagai H, Shibahara S, Matsushima R, Uchida H, Kanamori M, Nogata Y, Kamio M (2021) Hemolytic compound 3,7,11,15-tetramethyl-hexadecan-1,19-disulfate found in the invasive European sea squirt Ascidiella aspersa. Fish Sci 87:145–150
Namikoshi M, Fujiwara T, Nishikawa T, Ukai K (2006) Natural abundance 14C content of dibutyl phthalate (DBP) from three marine algae. Mar Drugs 4:290–297
Nordeng H (2009) Char ecology. Natal homing in sympatric populations of anadromous Arctic char Salvelinus alpinus (L.): roles of pheromone recognition. Ecol Freshw Fish 18:41–51
Nusnbaum M, Derby CD (2010) Effects of sea hare ink secretion and its escapin-generated components on a variety of predatory fishes. Biol Bull 218:282–292
Ogata H, Kitamura S, Takashima F (1994) Release of 13,14-dihydro-15-keto-prostaglandin-F2α, a sex pheromone, to water by cobitid loach following ovulatory stimulation. Fish Sci 60:143–148
Oguri Y, Watanabe M, Ishikawa T, Kamada T, Vairappan CS, Matsuura H, Kaneko K, Ishii T, Suzuki M, Yoshimura E, Yasuyuki N, Tatsufumi O (2017) New marine antifouling compounds from the red alga Laurencia sp. Mar Drugs 15:267
Okada H, Sakai DK, Sugiwaka K (1978) Chemical stimulus on the reproductive behavior of the pont smelt. Sci Rep Hokkaido Fish Hatch 33:89–99
Okamura A, Yamada Y, Mikawa N, Horie N, Tsukamoto K (2020) Dietary supplementation with chitin hydrolysates for Anguilla japonica leptocephali. Fish Sci 86:685–692
Okita K, Yamazaki H, Sakiyama K, Yamane H, Niina S, Takatani T, Arakawa O, Sakakura Y (2013) Puffer smells tetrodotoxin. Ichthyol Res 60:386–389
Olsén KH, Bjerselius R, Mayer I, Kindahl H (2001) Both ovarian fluid and female urine increase sex steroid hormone levels in mature Atlantic salmon (Salmo salar) male parr. J Chem Ecol 27:2337–2349
Olsén KH, Johansson A, Bjeriselius R, Mayer I, Kindhal H (2002) Mature Atlantic salmon (Salmo salar L.) male parr are attracted to ovulated female urine but not to ovarian fluid. J Chem Ecol 28:29–40
Olsén KH, Sawisky G, Stacey N (2006) Endocrine and milt responses of male crucian carp (Carassius carassius L.) to periovulatory females under field conditions. Gen Comp Endocrinol 149:294–302
Ortega Á, Zhulin IB, Krell T (2017) Sensory repertoire of bacterial chemoreceptors. Microbiol Mol Biol Rev 81:e00033-e17
Oyama T, Momohara Y, Yano H, Kamio M, Fujiyama N, Nagayama T (2020) Sex recognition and agonistic strategies of male and female crayfish. Behaviour 157:575–596
Pagett HE, Abrahams JL, Bones J, O’Donoghue N, Marles-Wright J, Lewis RJ, Harris JR, Caldwell GS, Rudd PM, Clare AS (2012) Structural characterisation of the N-glycan moiety of the barnacle settlement-inducing protein complex (SIPC). J Exp Biol 215:1192–1198
Painter SD, Clough B, Garden RW, Sweedler JV, Nagle GT (1998) Characterization of Aplysia attractin, the first water-borne peptide pheromone in invertebrates. Biol Bull 194:120–131
Paul VJ, Ritson-Williams R, Sharp K (2011) Marine chemical ecology in benthic environments. Nat Prod Rep 28:345–387
Pawlik JR (1993) Marine invertebrate chemical defenses. Chem Rev 93:1911–1922
Pennings SC, Paul VJ (1993) Sequestration of dietary secondary metabolites by three species of sea hares: location, specificity and dynamics. Mar Biol 117:535–546
Pickett JA, Woodcock CM, Midega CAO, Khan ZR (2014) Push–pull farming systems. Curr Opin Biotechnol 26:125–132
Poling KR, Fraser EJ, Sorensen PW (2001) The three steroidal components of the goldfish preovulatory pheromone signal evoke different behaviors in males. Comp Biochem Physiol B Biochem Mol Biol 129:645–651
Poulin RX, Lavoie S, Siegel K, Gaul DA, Weissburg MJ, Kubanek J (2018) Chemical encoding of risk perception and predator detection among estuarine invertebrates. Proc Natl Acad Sci USA 115:662–667
Poulson KL, Sieg RD, Kubanek J (2009) Chemical ecology of the marine plankton. Nat Prod Rep 26:729–745
Puglisi MP, Sneed JM, Sharp KH, Ritson-Williams R, Paul VJ (2014) Marine chemical ecology in benthic environments. Nat Prod Rep 31:1510–1553
Puglisi MP, Sneed JM, Ritson-Williams R, Young R (2019) Marine chemical ecology in benthic environments. Nat Prod Rep 36:410–429
Qian P-Y, Li Z, Xu Y, Li Y, Fusetani N (2015) Mini-review: marine natural products and their synthetic analogs as antifouling compounds: 2009–2014. Biofouling 31:101–122
Reverter M, Tapissier-Bontemps N, Lecchini D, Banaigs B, Sasal P (2018) Biological and ecological roles of external fish mucus: a review. Fishes 3:41
Roggatz CC, Lorch M, Hardege JD, Benoit DM (2016) Ocean acidification affects marine chemical communication by changing structure and function of peptide signalling molecules. Glob Chang Biol 22:3914–3926
Rolen SH, Sorensen PW, Mattson D, Caprio J (2003) Polyamines as olfactory stimuli in the goldfish Carassius auratus. J Exp Biol 206:1683–1696
Ross E, Behringer D (2019) Changes in temperature, pH, and salinity affect the sheltering responses of Caribbean spiny lobsters to chemosensory cues. Sci Rep 9:4375
Roy JS, Poulson-Ellestad KL, Sieg RD, Poulin RX, Kubanek J (2013) Chemical ecology of the marine plankton. Nat Prod Rep 30:1364–1379
Ryan EP (1966) Pheromone: evidence in a decapod crustacean. Science 151:340–341
Saha M, Berdalet E, Carotenuto Y, Fink P, Harder T, John U, Not F, Pohnert G, Potin P, Selander E, Vyverman W, Wichard T, Zupo V, Steinke M (2019) Using chemical language to shape future marine health. Front Ecol Environ 17:530–537
Sakata K, Sakura T, Ina K (1988) Algal phagostimulants for marine herbivorous gastropods. J Chem Ecol 14:1405–1416
Saraiva JL, Keller-Costa T, Hubbard PC, Rato A, Canario AVM (2017) Chemical diplomacy in male tilapia: urinary signal increases sex hormone and decreases aggression. Sci Rep 7:7636
Sato K, Suzuki N (2001) Whole-cell response characteristics of ciliated and microvillous olfactory receptor neurons to amino acids, pheromone candidates and urine in rainbow trout. Chem Senses 26:1145–1156
Sato K, Shoji T, Ueda H (2000) Olfactory discriminating ability of lacustrine sockeye and masu salmon in various freshwaters. Zool Sci 17:313–317
Sato T, Goshima S (2006) Impacts of male-only fishing and sperm limitation in manipulated populations of an unfished crab, Hapalogaster dentata. Mar Ecol Prog Ser 313:193–204
Sato T, Goshima S (2007) Female choice in response to risk of sperm limitation by the stone crab, Hapalogaster dentata. Anim Behav 73:331–338
Savoca MS, Wohlfeil ME, Ebeler SE, Nevitt GA (2016) Marine plastic debris emits a keystone infochemical for olfactory foraging seabirds. Sci Adv 2:e1600395
Scardino AJ, de Nys R (2011) Mini review: Biomimetic models and bioinspired surfaces for fouling control. Biofouling 27:73–86
Schaum CE, Batty R, Last KS (2013) Smelling danger—alarm cue responses in the polychaete Nereis (Hediste) diversicolor (Müller, 1776) to potential fish predation. PLOS ONE 8:e77431
Schmidt M, Derby CD (2005) Non-olfactory chemoreceptors in asymmetric setae activate antennular grooming behavior in the Caribbean spiny lobster Panulirus argus. J Exp Biol 208:233–248
Schmidt M, Chien H, Tadesse T, Johns ME, Derby CD (2006) Rosette-type tegumental glands associated with aesthetasc sensilla in the olfactory organ of the Caribbean spiny lobster, Panulirus argus. Cell Tissue Res 325:369–395
Schwartz ER, Poulin RX, Mojib N, Kubanek J (2016) Chemical ecology of marine plankton. Nat Prod Rep 33:843–860
Scott AM, Zhang Z, Jia L, Li K, Zhang Q, Dexheimer T, Ellsworth E, Ren J, Chung-Davidson YW, Zu Y, Neubig RR, Li W (2019) Spermine in semen of male sea lamprey acts as a sex pheromone. PLoS Biol 17:e3000332
Scott AP, Ellis T (2007) Measurement of fish steroids in water—a review. Gen Comp Endocrinol 153:392–400
Scott AP, Liley NR, Vermeirssen ELM (1994) Urine of reproductively mature female rainbow trout, Oncorhynchus mykiss (Walbaum), contains a priming pheromone which enhances plasma levels sex steroids and gonadotrophin II in males. J Fish Biol 44:131–147
Seuront L (2018) Microplastic leachates impair behavioural vigilance and predator avoidance in a temperate intertidal gastropod. Biol Lett 14:20180453
Shabani S, Kamio M, Derby CD (2008) Spiny lobsters detect conspecific blood-borne alarm cues exclusively through olfactory sensilla. J Exp Biol 211:2600–2608
Shah MD, Venmathi Maran BA, Haron FK, Ransangan J, Ching FF, Shaleh SRM, Shapawi R, Yong YS, Ohtsuka S (2020) Antiparasitic potential of Nephrolepis biserrata methanol extract against the parasitic leech Zeylanicobdella arugamensis (Hirudinea) and LC-QTOF analysis. Sci Rep 10:22091
Sherker ZT, Ellrich JA, Scrosati RA (2017) Predator-induced shell plasticity in mussels hinders predation by drilling snails. Mar Ecol Prog Ser 573:167–175
Shoji T, Ueda H, Ohgami T, Sakamoto T, Katsuragi Y, Yamauchi K, Kurihara K (2000) Amino acids dissolved in stream water as possible home stream odorants for masu salmon. Chem Senses 25:533–540
Shoji T, Yamamoto Y, Nishikawa D, Kurihara K, Ueda H (2003) Amino acids in stream water are essential for salmon homing migration. Fish Physiol Biochem 28:249–251
Siefkes MJ (2017) Use of physiological knowledge to control the invasive sea lamprey (Petromyzon marinus) in the Laurentian Great Lakes. Conserv Physiol 5:cox031
Siefkes MJ, Bergstedt RA, Twohey MB, Li W (2003) Chemosterilization of male sea lampreys (Petromyzon marinus) does not affect sex pheromone release. Can J Fish Aquat Sci 60:23–31
Siefkes MJ, Winterstein SR, Li W (2005) Evidence that 3-keto petromyzonol sulphate specifically attracts ovulating female sea lamprey, Petromyzon marinus. Anim Behav 70:1037–1045
Sieg RD, Poulson-Ellestad KL, Kubanek J (2011) Chemical ecology of the marine plankton. Nat Prod Rep 28:388–399
Smee DL, Weissburg MJ (2006) Clamming up: Environmental forces diminish the perceptive ability of bivalve prey. Ecology 87:1587–1598
Smith SG, Chang ES (2007) Molting and growth. In: Kennedy VS, Cronin LE (eds) The Blue Crab Callinectes sapidus. Maryland Sea Grant College, College Park, pp 197–254
Söffker M, Tyler CR (2012) Endocrine disrupting chemicals and sexual behaviors in fish—a critical review on effects and possible consequences. Crit Rev Toxicol 42:653–668
Sorensen PW, Johnson NS (2016) Theory and application of semiochemicals in nuisance fish control. J Chem Ecol 42:698–715
Sorensen PW, Wisenden BD (2015) Fish pheromones and related cues. Wiley-Blackwell, Hoboken
Sorensen PW, Hara TJ, Stacey NE, Goetz FW (1988) F-prostaglandins function as potent olfactory stimulants that comprise the postovulatory female sex pheromone in goldfish. Biol Reprod 39:1039–1050
Sorensen PW, Stacey NE, Chamberlain KJ (1989) Differing behavioral and endocrinological effects of two female sex pheromones on male goldfish. Horm Behav 23:317–332
Sorensen PW, Fine JM, Dvornikovs V, Jeffrey CS, Shao F, Wang J, Vrieze LA, Anderson KR, Hoye TR (2005a) Mixture of new sulfated steroids functions as a migratory pheromone in the sea lamprey. Nat Chem Biol 1:324–328
Sorensen PW, Pinillos M, Scott AP (2005b) Sexually mature male goldfish release large quantities of androstenedione into the water where it functions as a pheromone. Gen Comp Endocrinol 140:164–175
Sorensen PW, Appelt C, Stacey NE, Goetz FW, Brash AR (2018) High levels of circulating prostaglandin F2α associated with ovulation stimulate female sexual receptivity and spawning behavior in the goldfish (Carassius auratus). Gen Comp Endocrinol 267:128–136
Souza JCR, Strüssmann CA, Takashima F, Satoh H, Sekine S, Shima Y, Matsuda H (2010) Oral and integumental uptake of free exogenous glycine by the Japanese spiny lobster Panulirus japonicus phyllosoma larvae. J Exp Biol 213:1859–1867
Stacey N, Kobayashi M (1996) Androgen induction of male sexual behaviors in female goldfish. Horm Behav 30:434–445
Stacey N, Liley N (1974) Regulation of spawning behaviour in the female goldfish. Nature 247:71–72
Stacey NE, Peter RE (1979) Central action of prostaglandins in spawning behaviour of female goldfish. Physiol Behav 22:1191–1196
Stacey N, Sorensen P (2006) Reproductive pheromones. In: Katherine AS, Rod WW, Sigal B (eds) Fish physiology. Academic Press, New York, pp 359–412
Stacey N, Sorensen P, Van Der Kraak G, Dulka J (1989) Direct evidence that 17α, 20β-dihydroxy-4-pregnen-3-one functions as a goldfish primer pheromone: preovulatory release is closely associated with male endocrine responses. Gen Comp Endocrinol 75:62–70
Stacey NE, Zheng W, Cardwell J (1994) Milt production in common carp (Cyprinus carpio): stimulation by a goldfish steroid pheromone. Aquaculture 127:265–276
Stacey N, Fraser EJ, Sorensen P, Van Der Kraak G (2001) Milt production in goldfish: regulation by multiple social stimuli. Comp Biochem Physiol C Pharmacol Toxicol 130:467–476
Stensmyr MC, Erland S, Hallberg E, Wallén R, Greenaway P, Hansson BS (2005) Insect-like olfactory adaptations in the terrestrial giant robber crab. Curr Biol 15:116–121
Stepanyan R, Haley SB, McClintock TS (2005) Olfactory specific chymotrypsin-like serine protease from the aesthetasc tegumental gland of the lobster, Homarus americanus. Cell Tissue Res 322:321–330
Sveinsson T, Hara TJ (1995) Mature males of Arctic charr, Salvelinus alpinus, release F-type prostaglandins to attract conspecific mature females and stimulate their spawning behaviour. Environ Biol Fishes 42:253–266
Tabor CW, Tabor H (1984) Polyamines. Annu Rev Biochem 53:749–790
Tachibana K, Sakaitani M, Nakanishi K (1985) Pavoninins, shark-repelling and ichthyotoxic steroid N-acetylglucosaminides from the defense secretion of the sole Pardachirus pavoninus (Soleidae). Tetrahedron 41:1027–1037
Takeuchi T (2014) Progress on larval and juvenile nutrition to improve the quality and health of seawater fish: a review. Fish Sci 80:389–403
Takeuchi T, Haga Y (2015) Development of microparticulate diets with special reference to Pacific bluefin tuna, abalone, and Japanese spiny lobster: a review. Fish Sci 81:591–600
Talbot P, Demers D (1993) Tegumental glands of Crustacea. In: Michael NH, John AF (eds) The crustacean integument: morphology and biochemistry. CRC Press, New York, pp 151–191
Tebben J, Motti CA, Siboni N, Tapiolas DM, Negri AP, Schupp PJ, Kitamura M, Hatta M, Steinberg PD, Harder T (2015) Chemical mediation of coral larval settlement by crustose coralline algae. Sci Rep 5:10803
Teeter J (1980) Pheromone communication in sea lampreys (Petromyzon marinus): Implications for population management. Can J Fish Aquat Sci 37:2123–2132
Teytaud AR (1971) The laboratory studies of sex recognition in the blue crab Callinectes sapidus Rathbun. Univ Miami Sea Grant Tech Bull 15:1–63
Thiel M, Breithaupt T (2010) Chemical communication in crustaceans: research challenges for the twenty-first century. In: Thiel M, Breithaupt T (eds) Chemical communication in crustaceans. Springer, New York, pp 3–22
Tian C, Ni J, Chang F, Liu S, Xu N, Sun W, Xie Y, Guo Y, Ma Y, Yang Z (2016) Bio-Source of di-n-butyl phthalate production by filamentous fungi. Sci Rep 6:19791
Ting J, Snell T (2003) Purification and sequencing of a mate-recognition protein from the copepod Tigriopus japonicus. Mar Biol 143:1–8
Tintillier F, Moriou C, Petek S, Fauchon M, Hellio C, Saulnier D, Ekins M, Hooper JNA, Al-Mourabit A, Debitus C (2020) Quorum sensing inhibitory and antifouling activities of new bromotyrosine metabolites from the Polynesian sponge Pseudoceratina n. sp. Mar Drugs 18:272
Ueda H (2011) Physiological mechanism of homing migration in Pacific salmon from behavioral to molecular biological approaches. Gen Comp Endocrinol 170:222–232
Utermann C, Blumel M, Busch K, Buedenbender L, Lin YP, Haltli BA, Kerr RG, Briski E, Hentschel U, Tasdemir D (2020) Comparative microbiome and metabolome analyses of the marine tunicate Ciona intestinalis from native and invaded habitats. Microorganisms 8:2022
Van Den Hurk R, Schoonen WGEJ, Van Zoelen GA, Lambert JGD (1987) The biosynthesis of steroid glucuronides in the testis of the zebrafish, Brachydanio rerio, and their pheromonal function as ovulation inducers. Gen Comp Endocrinol 68:179–188
Vermeirssen ELM, Scott AP (2001) Male priming pheromone is present in bile, as well as urine, of female rainbow trout. J Fish Biol 58:1039–1045
Vermeirssen ELM, Scott AP, Liley NR (1997) Female rainbow trout urine contains a pheromone which causes a rapid rise in plasma 17,20β-dihydoroxy-4-pregnen-3-one levels and milt amounts in males. J Fish Biol 50:107–119
Vrieze LA, Bjerselius R, Sorensen PW (2010) Importance of the olfactory sense to migratory sea lampreys Petromyzon marinusseeking riverine spawning habitat. J Fish Biol 76:949–964
Wagner CM, Jones ML, Twohey MB, Sorensen PW (2006) A field test verifies that pheromones can be useful for sea lamprey (Petromyzon marinus) control in the Great Lakes. Can J Fish Aquat Sci 63:475–479
Wagner CM, Hanson JE, Meckley TD, Johnson NS, Bals JD (2018) A simple, cost-effective emitter for controlled release of fish pheromones: development, testing, and application to management of the invasive sea lamprey. PLOS ONE 13:e0197569
Wakabayashi K, Tanaka Y, Phillips BF (2019) Culture of slipper lobster larvae (Decapoda: Achelata: Scyllaridae) fed jellyfish as food. In: Radhakrishnan EV, Bruce FP, Gopalakrishnan A (eds) Lobsters: biology, fisheries and aquaculture. Springer, Singapore, pp 519–540
Wakimoto T (2017) Toward the dark matter of natural products. Chem Rec 17:1124–1134
Wakimoto T, Abe I (2012) Labile natural products. Medchemcomm 3:866–870
Weissburg MJ (2012) Death from downstream: chemosensory navigation and predator-prey processes. In: Brönmark C, Hansson L-A (eds) Chemical ecology in aquatic systems. Oxford University Press, New York, pp 96–110
Weissburg MJ, Zimmer-Faust RK (1991) Ontogeny versus phylogeny in determining patterns of chemoreception: initial studies with fiddler crabs. Biol Bull 181:205–215
Weissburg MJ, Zimmer-Faust RK (1994) Odor plumes and how blue crabs use them in finding prey. J Exp Biol 197:349–375
Weissburg M, Atkins L, Berkenkamp K, Mankin D (2012) Dine or dash? Turbulence inhibits blue crab navigation in attractive–aversive odor plumes by altering signal structure encoded by the olfactory pathway. J Exp Biol 215:4175–4182
Weissburg MJ, Smee DL, Ferner MC (2014) The sensory ecology of non-consumptive predator effects. Am Nat 184:141–157
Weissburg M, Poulin R, Kubanek J (2016) You are what you eat: a metabolomics approach to understanding prey responses to diet-dependent chemical cues released by predators. J Chem Ecol 42:1037–1046
Wildlife Division, Natural Environment Bureau (2004) IV: Examples of countermeasures for black bass and bluegill in various regions. In: Ministry of the Environment (ed) Impact of black bass and bluegill on native community and ecosystem and their control. Japan Wildlife Research Center, Tokyo, pp 61–150 (in Japanese)
Wisby WJ, Hasler AD (1954) Effect of olfactory occlusion on migrating silver salmon (O. kisutch). J Fish Res Board Can 11:472–478
Wishart DS, Tzur D, Knox C, Eisner R, Guo AC, Young N, Cheng D, Jewell K, Arndt D, Sawhney S (2007) HMDB: the human metabolome database. Nucleic Acids Res 35:D521–D526
Wolfe KD, Wainwright ML, Smee DL, Mozzachiodi R (2016) Eat or be eaten? Modifications of Aplysia californica feeding behaviour in response to natural aversive stimuli. Anim Behav 120:123–133
Wyatt TD (2014) Pheromones and animal behavior: chemical signals and signatures. Cambridge University Press, Cambridge
Wyatt TD (2017) Primer pheromones. Curr Biol 27:R739–R743
Wyatt TD, Hardege JD, Terschak J (2014) Ocean acidification foils chemical signals. Science 346:176–176
Yabuki Y, Koide T, Miyasaka N, Wakisaka N, Masuda M, Ohkura M, Nakai J, Tsuge K, Tsuchiya S, Sugimoto Y, Yoshihara Y (2016) Olfactory receptor for prostaglandin F2α mediates male fish courtship behavior. Nat Neurosci 19:897–904
Yamamoto Y, Ishizawa S, Ueda H (2008) Effects of amino acid mixtures on upstream selective movement of four Pacific salmon. Cybium 32:57–58
Yamamoto Y, Hino H, Ueda H (2010) Olfactory imprinting of amino acids in lacustrine sockeye salmon. PLOS ONE 5:e8633
Yamazaki F (1976) Application of hormones in fish culture. J Fish Res Board Can 33:948–958
Yamazaki F (1990) The role of urine in sex discrimination in the goldfish, Carassius auratus L. Bull Fac Fish Hokkaido Univ 41:155–161
Yamazaki F, Watanabe K (1979) The role of steroid hormones in sex recognition during spawning behaviour of the goldfish, Carassius auratus L. Proc Indian Natl Sci Acad (B Biol Sci) 45:505–511
Yambe H (2013) Roles of pheromones in reproductive behaviors. In: Munakata A, Kobayashi M, Arimoto T (eds) Behavioral study in fish and its implication for stock management, I control mechanisms of fish behavior. Suisangaku Series 176. Kouseisyakouseikaku, Tokyo, pp 28–46
Yambe H, Yamazaki F (2000) Urine of ovulated female masu salmon attracts immature male parr treated with methyltestosterone. J Fish Biol 57:1058–1064
Yambe H, Yamazaki F (2001a) Species-specific releaser effect of urine from ovulated female masu salmon and rainbow trout. J Fish Biol 59:1455–1464
Yambe H, Yamazaki F (2001b) A releaser pheromone that attracts methyltestosterone treated immature fish in the urine of ovulated female rainbow trout. Fish Sci 67:214–220
Yambe H, Yamazaki F (2006) Transitions of urine flow rate and kidney-somatic index in mature masu salmon. Fish Sci 72:1048–1053
Yambe H, Shindo M, Yamazaki F (1999) A releaser pheromone that attractsmales in the urine of mature female masu salmon. J Fish Biol 55:158–171
Yambe H, Kitamura S, Kamio M, Yamada M, Matsunaga S, Fusetani N, Yamazaki F (2006a) L-Kynurenine, an amino acid identified as a sex pheromone in the urine of ovulated female masu salmon. Proc Natl Acad Sci USA 103:15370–15374
Yambe H, Yamada M, Yamazaki F (2006b) Responses of immature male masu salmon parr to the urine of mature males. Ichthyol Res 53:182–184
Yambe H, Konno S, Kitamura S (2008) Urine of ovulated female masu salmon (Oncorhynchus masou) contains a priming pheromone which increases plasma 17,20 β-dihydroxy-4-pregnene-3-one level in mature male parr. Cybium 32:307–307
Yang H, Johnson PM, Ko K-C, Kamio M, Germann MW, Derby CD, Tai PC (2005) Cloning, characterization and expression of escapin, a broadly antimicrobial FAD-containing L-amino acid oxidase from ink of the sea hare Aplysia californica. J Exp Biol 208:3609–3622
Yano H, Kamio M, Nagai H (2016) The molting biomarker metabolite N-acetylglucosamino-1, 5-lactone in female urine of the helmet crab Telmessus cheiragonus. Biol Bull 230:143–151
Yashiroda Y, Yoshida M (2019) Intraspecies cell-cell communication in yeast. FEMS Yeast Res 19:foz71
Yoshihara Y (2014) Zebrafish olfactory system. In: Mori K (ed) The olfactory system. Springer, Tokyo, pp 71–96
Yoshino T, Kishimoto H (2008) Plotosus japonicus, a new eeltail catfish (Siluriformes: Plotosidae) from Japan. Bull Nat Sci Mus (Japan) Ser A 2:1–11
Yu Z-L, Hu N, Yang M-J, Song H, Hu Z, Wang X-L, Zhou C, Zhang Z-X, Zhang T (2019) Environmental water flow can boost foraging success of the juvenile rapa whelk Rapana venosa (Muricidae) in aquaculture tanks with still or flowing water: indication of chemosensory foraging. Aquaculture 513:734392
Zeeck E, Hardege J, Bartels-Hardege H, Wesselmann G (1988) Sex pheromone in a marine polychaete: determination of the chemical structure. J Exp Zool 246:285–292
Zettler ER, Mincer TJ, Amaral-Zettler LA (2013) Life in the “plastisphere”: microbial communities on plastic marine debris. Environ Sci Technol 47:7137–7146
Zhang C, Hara TJ (2009) Lake char (Salvelinus namaycush) olfactory neurons are highly sensitive and specific to bile acids. J Comp Physiol A 195:203–215
Zhang C, Brown SB, Hara TJ (2001) Biochemical and physiological evidence that bile acids produced and released by lake char (Salvelinus namaycush) function as chemical signals. J Comp Physiol B 171:161–171
Zhang D, Terschak JA, Harley MA, Lin J, Hardege JD (2011) Simultaneously hermaphroditic shrimp use lipophilic cuticular hydrocarbons as contact sex pheromones. PLOS ONE 6:e17720
Zhang D, Liu X, Harley MA, Hardege JD (2020) Uridine-5’-tri-phosphate is a candidate component of the soluble sex pheromone bouquet in a marine shrimp, Lysmata wurdemanni. Mar Ecol Prog Ser 640:139–146
Zheng W, Strobeck C, Stacey N (1997) The steroid pheromone 4-pregnen-17, 20ss-diol-3-one increases fertility and paternity in goldfish. J Exp Biol 200:2833–2840
Zimmer RK, Commins JE, Browne KA (1999) Regulatory effects of environmental chemical signals on search behavior and foraging success. Ecology 80:1432–1446
Zimmer RK, Ferrier GA, Kim SJ, Ogorzalek Loo RR, Zimmer CA, Loo JA (2017) Keystone predation and molecules of keystone significance. Ecology 98:1710–1721
Zimmer-Faust RK (1993) ATP: A potential prey attractant evoking carnivory. Limnol Oceanogr 38:1271–1275
Acknowledgements
We thank Dr. Charles Derby and the anonymous reviewers for reading this manuscript and for valuable discussions; MK was supported by JSPS KAKENHI (Grant no. 20K06235) and Florida Sea Grant (Grant no. SI-2017-0062). YH was supported by JSPS KAKENHI (Grant no. 21688016).
Funding
Published with support by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant no. JP19HP2002.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The error in the affiliation has been corrected.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kamio, M., Yambe, H. & Fusetani, N. Chemical cues for intraspecific chemical communication and interspecific interactions in aquatic environments: applications for fisheries and aquaculture. Fish Sci 88, 203–239 (2022). https://doi.org/10.1007/s12562-021-01563-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-021-01563-0