Abstract
The Great East Japan Earthquake and accompanying tsunamis occurred on 11 March 2011, causing huge damage to marine organisms. The invasive naticid gastropod Laguncula pulchella (Euspira fortunei), which was introduced with the imported clam Ruditapes philippinarum from China and Korea, survived the earthquake. The “growth break line” observed on the shell surface in over 90% of the individuals collected in Matsushima Bay and Matsukawa-ura Lagoon after the tsunamis was examined by scanning electron microscopy. Each shell presented three layers before and three after the growth break. However, the fracture surface consisted of five layers—two prismatic and three crossed lamellar—around the growth break line. This suggests that shell formation temporarily ceased following the tsunamis and that the five-layered shell may have developed in response to the stress caused by the tsunamis. The newly formed middle layer became thinner after the “tsunami break,” which may be the result of a rapid change in the mineralization process, including rapid shell growth and/or repair. These results suggest that the damage to and forcible removal of habitats by the tsunamis was stressful for L. pulchella. A decrease in or the cessation of shell formation after a tsunami may be a common phenomenon in mollusks that inhabit tidal flats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The moon snail Laguncula pulchella (Torigoe and Inaba 2011), formally known as Euspira fortunei (Habe and Okutani 1983; Saito 2000; Okoshi 2004), which is mainly distributed in China, Korea, and southwest Japan, lives on muddy bottoms from the intertidal zone to 15 m deep. This is an alien species in northern Japan and was introduced with the imported Manila clam Ruditapes philippinarum from China and Korea for seeding and shellfish gathering (Okoshi 2004). As L. pulchella prefers to prey on the Manila clam (Chiba and Sato 2012; Sato et al. 2012), there was a concern that it would cause great damage to clam production in northern Japan (Okoshi 2007), including in Miyagi and Fukushima prefectures (Tomiyama et al. 2011; Okoshi and Sato-Okoshi 2011; Abe et al. 2017).
On 11 March 2011, the magnitude-9 Great East Japan Earthquake occurred along the Pacific coast of northern Japan. It caused massive tsunamis that struck northern Japan. The sandy and rocky shore habitats where marine organisms, including mollusks, live in the intertidal zone were drastically changed by the tsunamis, and marine ecosystems suffered great damage (Miura et al. 2012, 2017; Okoshi 2013, 2015, 2016; Suzuki 2013; Tamaki and Muraoka 2013; Takami et al. 2013; Urabe et al. 2013; Kawamura et al. 2014; Kanaya et al. 2014, 2015; Abe et al. 2015, 2017; Noda et al. 2016; Noda and Iwasaki 2017; Muraoka et al. 2017).
The impacts of the earthquake and subsequent tsunamis on commercially important mollusk species along the Tohoku coast such as abalones, oysters, and scallops have been of particular concern (Kawamura et al. 2014; Nagasawa et al. 2016; Onozato et al. 2016). In Matsukawa-ura Lagoon, Fukushima Prefecture, it has become impossible to catch Manila clams because of rumored damage caused by radioactivity from the Fukushima Daiichi Nuclear Power Plant. Even in areas distant from the power plant, e.g., Mangoku-ura Lagoon in Miyagi Prefecture, clam fishing has also become impossible due to the subsidence of the fishery grounds. While the production of these mollusks, apart from the Manila clam, has been gradually recovering since the earthquake (Tanabe 2013; Nagasawa et al. 2016), far less is known about the post-quake impact of the alien snail L. pulchella. Before the earthquake, we conducted a series of field surveys along the Pacific coast of the Tohoku district over several years to examine the impacts of the invasive snail L. pulchella on the coastal ecosystem of the region and the commercially important Manila clam resources (Okoshi 2004; Okoshi and Sato-Okoshi 2011; Tomiyama et al. 2011; Ohtsuki et al. 2016). The surveys covered coastal areas from the inner bay of Soma City, Fukushima Prefecture, to Rokkasyo Village, Aomori Prefecture. These data and samples obtained before the earthquake provided us with a unique opportunity to examine the effects of the 2011 Great East Japan Earthquake on the invasive snail L. pulchella in this area.
A mollusk’s shell potentially provides a record of environmental information in the form of bivalve shell growth bands, for example, which offer a precise chronological record as they change in response to environmental and physiological factors such as temperature (Nishida et al. 2012; Kubota et al. 2017), salinity (Schöne et al. 2013; Shirai et al. 2014), and spawning (Nishida et al. 2012). Pollard et al. (1977) reported that the land snail Helix pomatia presents an annual growth break in its shell, corresponding to the winter season. Although L. pulchella has been known to spawn in temperatures of < 20 °C from September to November (Okoshi and Sato-Okoshi 2011), the surface of the shell of L. pulchella is usually smooth, and no seasonal nor annual growth break has been observed on L. pulchella shells.
L. pulchella that survived the earthquake have been found in tidal flats, and a “growth break line” formed by a decrease in or the cessation of shell growth was observed on shell surfaces in Matsushima Bay and Matsukawa-ura Lagoon in northern Japan (Okoshi et al. 2014). It was reported that a large number of bivalve shells with defects in the shell margin were also observed after the earthquake (Okoshi 2013, 2015). Accordingly, it was suggested the growth break formed in response to the earthquake and tsunamis because of damage to and forcible removal from the habitat, regardless of the low temperature and spawning.
In the work reported in this paper, we focused on the invasive species L. pulchella, which inhabits the sandy intertidal zone, and described the short-term effects (within one year) of the Great East Japan Earthquake and the associated tsunamis on the shell growth of L. pulchella. We first observed and compared the shell microstructures (Taylor and Layman 1972; Carter 1980) of L. pulchella individuals collected before the earthquake to their microstructure after the earthquake. We also examined the formation process for the growth breaks observed on the fracture surfaces of shells after the earthquake. Finally, we examined the influence of the earthquake on shell formation by and the survival of L. pulchella. This is the first report on the impact of the 2011 Great East Japan Earthquake on marine alien species from the viewpoint of shell formation.
Materials and methods
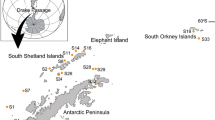
Specimens of adult L. pulchella (shell height 18–35 mm) were collected by hand from May to November 2011 at two tsunami-affected tidal flat stations that had experienced land subsidence caused by the 2011 earthquake (Fig. 1a). A station in Matsushima Bay (MB; 38°21.08′N, 141°03.34′E; maximum depth 4.0 m; water surface area 35.3 km2; maximum fluctuation of the tidal level 1.7 m; Fig. 1b) experienced a maximum inundation depth of 2.8 m during the tsunami and 41 cm of land subsidence (Kanaya et al. 2017). The area was covered by sandy and muddy deposits. Another station, in Matsukawa-ura Lagoon (ML; 37°48.46′N, 140°58.21′E; maximum depth 4.0 m; water surface area 6.5 km2; maximum fluctuation of the tidal level 1.5 m; Fig. 1c), experienced a maximum inundation depth of 5.3 m during the tsunami and 32 cm of land subsidence (Kanaya et al. 2017). Again, the area was covered by sandy and muddy deposits. Additionally, we checked for the presence of a growth break and compared the shell morphology before and after the earthquake, using the samples collected in MB in August 2008 to observe the shell morphology before the earthquake. Almost all tidal flats in Mangoku-ura Lagoon (38°25.05′N, 141°22.53′E; maximum depth 3.9 m; water surface area 7.4 km2; maximum fluctuation of the tidal level 1.6 m; Fig. 1d) have been lost due to ca. 80 cm of land subsidence since the earthquake (Okoshi 2015, 2016). Since October 2013, mountain sediment has been transferred to the sea to construct artificial tidal flats for Manila clam culture (Okoshi 2015). L. pulchella juveniles have been recruited in an artificial tidal flat since 2013. We collected L. pulchella individuals that were born after the earthquake and turned 3 years old in the artificial tidal flats in July 2016.
To observe the outer morphology and inner microstructure of each snail shell, we used a stereomicroscope (MZ16A, Leica Microsystems GmbH, Wetzlar, Germany), a biological microscope (DM 4000B, Leica Microsystems GmbH, Wetzlar, Germany), and a scanning electron microscope (SEM; 6390LV, JEOL Ltd., Tokyo, Japan). After removing the flesh, the shells were rinsed with water several times and then dried at room temperature (22 °C). Small pieces of shell were coated with Au (400 Å) using a deposition apparatus (SC-701AT, Sanyu Electron Co., Ltd., Tokyo, Japan), as per the standard procedure for imaging using SEM. The outer shell surface and fractured cross-section of each shell were imaged using SEM to observe its microstructure and architecture. The shell of L. pulchella consists of three layers (Okoshi and Sato-Okoshi 2011). For each individual, we measured the thicknesses of these three layers with different microstructures before and after the growth break and at points corresponding to 10 mm of growth from the break line (Fig. 2a) using the image software ImageJ v.1.49 (available as freeware at https://imagej.nih.gov/ij/). We performed stereomicroscopic analysis of the outer morphology of the shell for 845 (from MB) and 284 (from ML) individuals and 20 individuals from each site were analyzed via SEM. We also measured and compared the thicknesses of the shell layers in 10 individuals collected in May 2011 at the two stations. The value obtained by dividing the shell thickness by the shell height (ST/SH) to eliminate the effect of size differences on the analysis was expressed as the mean value ± SE and tested using one-way analysis of variance (one-way ANOVA) followed by the Tukey–Kramer multiple comparison test. p values of < 0.05 were considered to indicate statistically significant differences.
Laguncula pulchella shells with a straight growth break (a) and a jagged crack (b) collected in Matsukawa-ura Lagoon, June 2011. The black arrow indicates the position of the growth break. The white dotted line indicates the center of the body whorl. The rectangles indicate observation and measurement points before the growth break (right), after the growth break (middle), and at 10 mm of growth (left) after the break
Results
Shells collected after the earthquake showed changes in thickness, dents, and sometimes changes in color (Fig. 2a). They also displayed cracks around the aperture of the shell (Fig. 2b). Some individuals had shells that were broken into jagged edges. In addition, newly formed shell was observed at the aperture (Fig. 2b). Many individuals were observed to have one straight step (growth break line; arrow in Fig. 2a) on the shell. The color of the shell changed in the section after the step. Individuals with a step in the shell comprised more than 90% of the samples collected after the earthquake from both MB (99.3%; Fig. 3a) and ML (93.3%; Fig. 3b). In contrast, 23.5% of the specimens collected before the earthquake displayed a step in the shell (Fig. 3c). Among individuals that were born after the earthquake and collected from the artificial tidal flats in Mangoku-ura Lagoon, around 24.0% had a step in the shell (Fig. 3d).
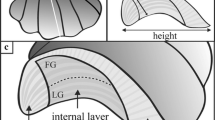
The shell of L. pulchella consisted of three layers: an outer prismatic layer and middle and inner crossed lamellar layers (Okoshi and Sato-Okoshi 2011; Figs. 4a–f). The middle and inner crossed lamellar layers had similar microstructures but different crystal growth directions. At the aperture, the shell consisted of two layers: the outer prismatic layer and the middle crossed lamellar layer (Figs. 4g, h). The thicknesses of shells 18–32 mm in height were revealed by SEM (shell height: MB 26.00 ± 4.42 mm, ML 23.80 ± 4.32 mm; shell thickness: MB 320.78 ± 86.12 μm, ML 372.53 ± 65.44 μm, N = 10). A thin periostracal layer covered the calcareous surface. Stereomicroscopic (Fig. 4a, c, e, g) and SEM (Fig. 4b, d, f, h) observations revealed that the shell surface was almost smooth. A growth break (arrows in Fig. 4i, j) was seen on the surfaces of the shells of L. pulchella individuals collected from MB and ML, as mentioned above.
Stereomicroscopic and SEM images of shells before (a, b) and after (c, d) a growth break, at a point corresponding to 10 mm of growth following the break (e, f), at the edge of the aperture of the shell (g, h), the shell fracture surface (i), and the outer surface at the growth break (j). The white dotted line indicates the transitional zone between different microstructures. P prismatic layer, MCL middle crossed lamellar layer, ICL inner crossed lamellar layer
The shell of L. pulchella normally consists of three layers. This was still true after a growth break had formed; however, the fracture surface consisted of five layers—two prismatic and three crossed lamellar layers (Fig. 5)—around the growth break.
SEM photographs of the shell fracture surface at the growth break. The white arrow indicates the position of the growth break. The white dotted line indicates the transitional zone between different microstructures. P prismatic layer, MCL middle crossed lamellar layer, ICL inner crossed lamellar layer
At each site, shell thickness changed after the growth break (Fig. 6a–d). The mean ST/SH value after the growth break (mean ± SE; MB 15.14 ± 0.69; ML 17.88 ± 1.52; N = 10) and that at the point corresponding to 10 mm of growth following the growth break (“10-mm growth point;” MB 16.88 ± 0.85; ML 19.26 ± 2.03, N = 10) were less than the mean ST/SH value before the growth break (MB 19.49 ± 0.54; ML 25.16 ± 1.59; N = 10) in MB and ML (Fig. 6a). A statistically significant difference between the mean ST/SH values before and after the growth break was only observed for the snails in MB (p < 0.05, one-way ANOVA followed by Tukey–Kramer test). The mean value of the outer prismatic layer was higher after the growth break (MB 2.28 ± 0.20; ML 4.06 ± 0.46; N = 10) than before the growth break (MB 1.38 ± 0.32; ML 2.05 ± 0.32; N = 10). There was a tendency for the value to increase further at the 10-mm growth point (MB 4.25 ± 0.57; ML 5.90 ± 0.58; N = 10) (Fig. 6b). A statistically significant difference was detected between the mean ST/SH value after the growth break (p < 0.05) in snails from ML or the value at the 10-mm growth point (p < 0.01) as compared with the mean ST/SH value before the growth break in snails from MB and ML, as well as between the mean ST/SH value after the growth break and that at the 10-mm growth point (p < 0.05) in snails from MB and ML. Conversely, the mean ST/SH value of the middle crossed lamellar layer was lower after the growth break (MB 4.48 ± 0.43; ML 5.41 ± 0.97; N = 10) than before the growth break (MB 11.04 ± 0.95; ML 12.83 ± 0.87; N = 10). This trend continued until the 10-mm growth point (MB 6.01 ± 0.67; ML 5.92 ± 1.00; N = 10) (Fig. 6c) in MB and ML snails. The mean ST/SH value after the growth break (p < 0.01) and at the 10-mm growth point (p < 0.01) were statistically significantly different from the mean ST/SH value before the growth break in MB and ML snails. The mean ST/SH value of the inner crossed lamellar layer before the growth break (MB 7.07 ± 0.54; ML 10.82 ± 1.04; N = 10) was almost the same as that after the growth break (MB 8.38 ± 0.57; ML 8.42 ± 1.31; N = 10) and that at the 10-mm growth point (MB 6.62 ± 0.80; ML 7.43 ± 0.96; N = 10) (Fig. 6d); there was no statistically significant difference between the MB and ML snails in the mean ST/SH value of the inner crossed lamellar layer before and after the growth break and at the 10-mm growth point.
Comparison of the snail samples from Matsushima Bay and Matsukawa-ura Lagoon in terms of shell thickness divided by shell height (ST/SH) values for all layers combined (a), the prismatic layer (b), the middle crossed lamellar layer (c), and the inner crossed lamellar layer (d) before and after the growth break and at the 10-mm growth point. Solid bars indicate standard errors. Asterisks (*p < 0.05, **p < 0.01) imply that the values indicated are significantly different (one-way ANOVA followed by the Tukey–Kramer test). n.s. not significant (p > 0.05)
Discussion
Most mollusks have a multilayered shell consisting of different microstructures. In addition, shell microstructural formation is affected by environmental and physiological factors (Carter 1980; Kennish 1980; Lutz and Rhoads 1980; Lutz and Clark 1984; Nishida et al. 2012). The shell of L. pulchella consists of three layers (Okoshi and Sato-Okoshi 2011). However, the shell in the vicinity of the aperture consisted of two layers without an inner crossed lamellar layer. The inner layer began to form several millimeters inward from the aperture in individuals with a shell height of 18–32 mm. Mollusc shell grows in two directions: in length and in thickness. Increasing the inner crossed lamellar layer thickens the shell, whereas increasing the outer prismatic layer and/or the middle crossed lamellar layer leads to shell elongation.
The L. pulchella shells observed in this work consisted of five layers just before the growth break (Fig. 5). Normally, the inner crossed lamellar layer forms after the middle crossed lamellar layer. However, at the growth break, the prismatic layer formed next to the middle crossed lamellar layer. After that, the middle crossed lamellar layer and inner crossed lamellar layer formed as usual (Fig. 5). L. pulchella individuals with shell margins that were cracked in a jagged fashion were thought to have received even greater damage than the individuals with a straight growth break line. Thus, it seems that shell formation temporarily stopped when the earthquake and tsunami struck. Unlike with bivalves, growth line analysis (e.g., Schöne et al. 2013) cannot be performed for the shells of many gastropods, including L. pulchella. Nishida et al. (2012) reported that shell growth of the blood cockle Anadara broughtonii was reduced in the low water temperature season. Sato (1997) reported that shell microgrowth of the venerid bivalve Dosinia japonica was low in the winter season as a reaction to seasonal changes in phytoplankton abundance. These results for bivalves were obtained by growth line analysis because the number of growth lines on a bivalve shell depends on daily tidal levels. However, gastropods (including L. pulchella) do not form daily growth lines. Therefore, we could not determine the length of shell formation cessation. The tsunami swept away many things, including debris and marine organisms, and many mollusks must have collided with debris during the tsunami. Such collisions can remove the periostracum from the margin of the shell or chip the shell. A newly formed periostracum formed after the earthquake, and three layers were formed in order inside the middle crossed lamellar layer. This is believed to be why five shell layers were sometimes observed. The size of the tsunami in ML was larger than that in MB. However, there does not seem to be a relationship between the size of the tsunami and the formation of a growth break.
Characteristic changes were also observed in the thickness of each layer by SEM. After the growth break, the total shell thickness decreased, the outer prismatic structure became thicker, the middle crossed lamellar layer became much thinner, and the inner crossed lamellar layer remained roughly the same thickness as before the growth break (Fig. 6). Shell structure is related both to physiological (e.g., spawning) and environmental (e.g., salinity, temperature) factors (Nishida et al. 2012). The prismatic structure, which is constructed from calcium carbonate and interprismatic organic material, is known to have elastic properties. Sato-Okoshi et al. (2010) reported that the shell of the pteropod Limacina has good elasticity, flexibility, and mechanical strength because of the way the shell microstructure, including the prismatic and crossed lamellar structures, is formed. The organic matrix is responsible for the elasticity of the outer prismatic layer, which helps the inner crossed lamellar layer to resist cracking and breaking. Taylor and Layman (1972) and Carter (1980) classified the microstructures of the shell at the SEM level and reported a range of mechanical properties and functions of bivalve shells. Li et al. (2017) also reviewed the mechanical features of the crossed lamellar structure. According to those reports, this structure was found to be the hardest but also the least elastic shell microstructure. Therefore, changes in the thickness of each layer formed after the growth break may be the result of rapid changes in the mineralization process, including quick shell growth to protect from debris collision and/or to implement rapid shell repair.
Because about 25% of the specimens collected before the earthquake displayed a growth break in their shell, environmental and physiological factors (e.g., typhoons, strong waves, crab predation, spawning) other than the tsunami are also likely to be involved in the formation of a growth break. In comparison, individuals with a growth break comprised more than 90% of the surviving samples collected after the earthquake. Based on these results, it appears that the effects of the earthquake often led to stepped and jagged shell fissures. A tsunami is considered to be a strong environmental stress that causes temporary growth suppression or cessation in mollusks. Therefore, we decided to call the step a “growth break” or “tsunami break.” It may be possible to find tsunami breaks in shells excavated from fossils and shell mounds.
L. pulchella is native to the coast of China and the Korean Peninsula, which is rarely subjected to tsunamis (Okoshi 2016), so this species is unlikely to be exposed to massive tsunamis in its native habitat. The 2011 Great East Japan Earthquake and the associated tsunamis are believed to have been the first such events that this species has experienced. Surviving individuals of L. pulchella were found at all of the sites observed in this study following the earthquake. This suggests that it may be tolerant of such disturbances, regardless of the size of the tsunami and the degree of land subsidence. Because tsunami breaks have been observed in both bivalves and gastropods, a decrease in or the cessation of shell formation after a tsunami may be a common phenomenon in mollusks that inhabit tidal flats.
References
Abe H, Kobayashi G, Sato-Okoshi W (2015) Impacts of the 2011 tsunami on the subtidal polychaete assemblage and the following recolonization in Onagawa Bay, northeastern Japan. Mar Environ Res 112:86–95
Abe H, Sato T, Iwasaki T, Wada T, Tomiyama T, Sato T, Hamaguchi M, Kajihara N, Kamiyama T (2017) Impact of the 2011 on the Manila clam Ruditapes philippinarum population and subsequent population recovery in Matsukawa-ura Lagoon, Fukushima, northeastern Japan. Region Stud Mar Sci 9:97–105
Carter JG (1980) Environmental and biological controls of bivalve shell mineralogy and microstructure. In: Rhoads DC, Lutz RA (eds) Skeletal growth of aquatic organisms: biological records of environmental change. Topics in geobiology series. Plenum, New York, pp 69–113
Chiba T, Sato S (2012) Size-selective predation and drillhole-site selectivity in Euspira fortunei (Gatropoda: Naticidae): implications for ecological and palaecological studies. J Mollusc Stud 78:205–212
Habe T, Okutani T (1983) The mollusks of Japan (sea snails). Gakken Holdings, Tokyo, p 80 (in Japanese)
Kanaya G, Maki H, Suzuki T, Sato-Okoshi W, Kikuchi E (2014) Tsunami-induced changes in a shallow brackish lagoon ecosystem (Gamo Lagoon) in Sendai Bay, Japan. Glob Environ Res 18:35–46
Kanaya G, Suzuki T, Kikuchi E (2015) Impacts of the 2011 tsunami on sediment characteristics and macrozoobenthic assemblages in a shallow eutrophic lagoon, Sendai Bay, Japan. PLoS One 10:e0135125
Kanaya G, Suzuki T, Kinoshita K, Matsumasa M, Yamada K, Seike K, Okoshi K, Miura O, Nakai S, Sato-Okoshi W, Kikuchi E (2017) Disaster-induced changes in coastal wetlands and soft-bottom habitats in eastern Japan—an overview on 2011 Great East Japan Earthquake. Biol Int SI36:62–80
Kawamura T, Tamami H, Hayakawa J, Won N, Muraoka D, Kurita Y (2014) Changes in abalone and sea urchin populations in rocky reef ecosystems on the Sanriku Coast damaged by the massive tsunami and other environmental changes associated with the Great East Japan Earthquake in 2011. Glob Environ Res 18:47–56
Kennish MJ (1980) Shell microgrowth analysis: Mercenaria mercenaria as a type example for research in population dynamics. In: Rhoads DC, Luts RA (eds) Skeletal growth of aquatic organisms: biological records of environmental change. Topics in geobiology series. Plenum, New York, pp 597–601
Kubota K, Shirai K, Murakami-Sugihara N, Seike K, Hori M, Tanabe K (2017) Annual shell growth pattern of the Stimpson’s hard clam Mercenaria stimpsoni as revealed by sclerochronological and oxygen stable isotope measurements. Palaeogeogr Palaeoclimat Palaeoecol 465:307–315. https://doi.org/10.1016/j.palaeo.2016.05.016
Li XW, Ji HM, Yang W, Zhang GP, Chen DL (2017) Mechanical properties of crossed-lamellar structures in biological shells: a review. J Mech Behav Biomed Mater 74:54–71
Lutz RA, Clark GR (1984) Seasonal and geographic variation in the shell microstructure of a salt marsh bivalve (Geukensia demissa (Dillwyn)). J Mar Res 42:943–956
Lutz RA, Rhoads DC (1980) Growth patterns within the molluscan shell: an overview. In: Rhoads DC, Lutz RA (eds) Skeletal growth of aquatic organisms: biological records of environmental change. Topics in geobiology series. Plenum, New York, pp 203–254
McKindsey DW, Landry T, O’Beirn FX, Davies IM (2007) Bivalve aquaculture and exotic species: a review of ecological considerations and management issues. J Shellfish Res 26:281–294
Miura O, Sasaki Y, Chiba S (2012) Destruction of population of Batillaria attramentaria (Caenogastropoda: Batillaridae) by tsunami waves of the 2011 Tohoku earthquake. J Mollusc Stud 78:377–380
Miura O, Kanaya G, Nakai S, Itoh H, Chiba S, Makino W, Nishimura T, Kojima S, Urabe J (2017) Ecological and genetic impact of the 2011 Tohoku Earthquake Tsunami on intertidal mud snails. Sci Rep 7:44375. https://doi.org/10.1038/srep44375
Muraoka D, Tamaki H, Takami H, Kurita Y, Kawamura T (2017) Effects of the 2011 Great East Japan Earthquake and tsunami on two kelp bed communities on the Sanriku coast. Fish Oceanogr 26:128–140
Nagasawa K, Takahashi D, Itoh N, Takahashi K, Osada M (2016) Growth of Japanese scallop Mizuhopecten yessoensis farmed by the ear-hanging metod between different water layers and estimation of the productivity for farmed scallops in Ogatsu Bay (northeastern Japan) following the 2011 Great East Japan Earthquake. Nippon Suisan Gakkaishi 82:321–329 (in Japanese with English abstract)
Nishida K, Ishimura T, Suzuki A, Sasaki T (2012) Seasonal changes in the shell microstructure of the bloody clam, Scapharca boughtonii (Mollusca: Bivalvia: Arcidae). Palaeogeogr Palaeoclim Palaeoecol 363–364:99–108. https://doi.org/10.1016/j.palaeo.2012.08.017
Noda T, Iwasaki A (2017) Rocky intertidal community: impact of earthquake and tsunami. Nippon Suisan Gakkaishi 83:677–680 (in Japanese)
Noda T, Iwasaki A, Fukaya K (2016) Recovery of rocky intertidal zonation: two years after the 2011 Great East Japan Earthquake. J Mar Biol Assoc UK 96:1549–1555
Ohtsuki H, Suzuki T, Kinoshita K, Kanaya G, Hirama T, Sato S, Shibata K, Okoshi K, Urabe J (2016) Genetic structures of Laguncula pulchella metapopulations along the northeast coast of Japan after the tsunamis caused by the Great East Japan Earthquake. In: Urabe J, Nakashizuka T (eds) Ecological impacts of tsunamis on coastal ecosystems. Springer, Cham, pp 209–221
Okoshi K (2004) Alien species introduced with imported clams: the clam-eating moon snail Euspira fortunei and other unintentionally introduced species. Japan J Benthol 59:74–82 (in Japanese with English abstract)
Okoshi K (2007) Unintentionally introduced species—the clam-eating moon snail Euspira fortunei. Nipp Suis Gakk 73:1129–1132 (in Japanese with English abstract)
Okoshi K (2013) Changes in the distribution and abundance of intertidal bivalves in sandy shore one and a half years after the Pacific coast of Tohoku Earthquake. J Jpn Soc Water Environ 36:44–48 (in Japanese)
Okoshi K (2015) Impact of repeating massive earthquakes on intetidal mollusk community in Japan. In: Ceccaldi H-J et al (eds) Marine productivity: disturbance and resilience of coastal socio-ecosystems. Springer, Berlin, pp 55–62
Okoshi K (2016) The effects of liquefaction, tsunami, and land subsidence on intertidal mollusks following the Great East Japan Earthquake. In: Urabe J, Nakashizuka T (eds) Ecological impacts of tsunamis on coastal ecosystems. Springer, Berlin, pp 165–178
Okoshi K, Sato-Okoshi W (2011) Euspira fortunei: biology and fisheries science of invasive species. Kouseisyakouseikaku, Tokyo, p 225 (in Japanese)
Okoshi K, Suzuki M, Maruyama Y, Shinohara W (2014) Traces of the earthquake and tsunami observed in shells. Monthly Chikyu 36:42–46 (in Japanese)
Onozato M, Nishigaki A, Okoshi K (2016) Polycyclic aromatic hydrocarbons in sediments and bivalves on the Pacific coast of Japan: influence of tsunami and fire. PLoS One 11(5):e0156447. https://doi.org/10.1371/journal.pone.0156447
Pollard E, Cooke AS, Welch JM (1977) The use of shell features in age determination of juvenile and adult Roman snails Helix pomatia. J Zool 183:269–279
Saito H (2000) Marine mollusks in Japan. Tokai University, Tokyo, pp 250–251 (in Japanese)
Sato S (1997) Shell microgrowth patterns of bivalves reflecting seasonal change of phytoplankton abundance. Paleontol Res 1:260–266
Sato S, Chiba T, Hasegawa H (2012) Long-term fluctuations in mollusk populations before and after the appearance of the alien predator Euspira fortunei on the Tona coast, Miyagi Prefecture, northern Japan. Fish Sci 78:589–595
Sato-Okoshi W, Okoshi K, Sasaki H, Akiha F (2010) Shell structure of two polar pelagic molluscs, Arctic Limacina helicina and Antarctic Limacina helicina antarctica forma antarctica. Polar Biol 33:1577–1583
Schöne BR, Radermacher P, Zhang Z, Jacob DE (2013) Crystal fabrics and element impurities (Sr/Ca, Mg/Ca, and Ba/Ca) in shells of Arctica islandica—implications for paleoclimate reconstructions. Palaeogeogr Palaeoclimat Palaeoecol 373:50–59
Shirai K, Schöne RB, Miyaji T, Radarmacher P, Krause AR Jr, Tanabe K (2014) Assessment of the mechanism of elemental incorporation into bivalve shells (Arctica islandica) based on elemental distribution at the microstructural scale. Geochim Cosmochim Acta 126:307–320
Suzuki T (2013) Natural environment of Matsukawa-ura Lagoon and the impacts of the earthquake disaster from the point of view of benthic animals. In: Maekawa S (ed) WWF Japan report on the nature and livelihood recovery project. A preliminary assessment of ecological and social-economic changes in selected areas affected by the Great East Japan Earthquake. WWF, Tokyo, pp 20–33 (in Japanese)
Takami H, Won N, Kawamura T (2013) Impacts of the 2011 mega-earthquake and tsunami on abalone Haliotis discus hannai and sea urchin Strongylocentrotus nudus populations at Oshika Peninsula, Miyagi, Japan. Fish Oceanogr 22(2):113–120
Tamaki H, Muraoka D (2013) Disturbances of tidal flat, Zostera and Eisenia bicyclis habitats by the Great East Japan Earthquake and their recovery in Miyagi Prefecture. Environ Conserv Eng 42:558–563 (in Japanese with English abstract)
Tanabe T (2013) Impact of the Great Eastern Japan Earthquake and efforts for restoration on Japanese oyster Crassostrea gigas farming. Nippon Suisan Gakkaishi 79:721–723 (in Japanese)
Taylor JD, Layman MA (1972) The mechanical properties of bivalve (Mollusca) shell structures. Palaeont 15:73–87
Tomiyama T, Suzuki T, Sato T, Kato Y, Kameiwa S, Sugibayashi Y, Okoshi K (2011) Unintentional introduction and the distribution of the nonindigenous moon snail Euspira fortunei in Matsukawaura Lagoon, Japan. Nippon Suisan Gakkaishi 77:1020–1026 (in Japanese with English abstract)
Torigoe K, Inaba A (2011) Revision on the classification of recent Naticidae. Bulle Nishinomiya Shell Museum 7:133 (in Japanese)
Urabe J, Suzuki T, Nishita T, Makino W (2013) Immediate ecological impacts of the 2011 Tohoku earthquake tsunami on intertidal flat communities. PLoS One 8:e62779
Acknowledgements
We are grateful to members of the Laboratory of Marine Ecology, Graduate School of Environmental Science, Toho University, for their cooperation and assistance during sample collection and field observations. We are also grateful to Prof. Koji Inoue of the Atmosphere and Ocean Research Institute, The University of Tokyo, and to two anonymous reviewers who critically reviewed the manuscript and made important suggestions. This study was partially supported by a research grant from the Tohoku Ecosystem-Associated Marine Sciences (TEAMS) research program of the Ministry of Education, Culture, Sports, Science and Technology (MEXT).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Suzuki, M., Okoshi, K. The “tsunami break:” impact of the Great East Japan Earthquake and the accompanying tsunamis on the shell growth of the invasive clam-eating snail Laguncula pulchella. Fish Sci 84, 485–494 (2018). https://doi.org/10.1007/s12562-018-1197-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-018-1197-2