Abstract
“Burnt meat” is a term used to describe the white (pale, grainy, exudative) muscle of yellowtail or tuna. It (with lightness parameter, L* ≥ 55) was observed after 2 h storage in the suffocate in air (SA) 29°C group and after 4 h storage in the spinal cord destruction (SCD) 29°C group. In the SA 17°C group, burnt meat was also observed after 4 h storage. In contrast, the meat in the SCD 17°C group was normal until after 12 h storage. The myosin heavy chain (MHC) was more degraded than the other myofibrillar proteins, and some protein bands increased in the burnt meat. The protease that leads to the degradation of MHC was investigated using myofibrils from the meat. EDTA completely suppressed the degradation, indicating that a myofibril-bound EDTA-sensitive protease (MBESP) may exist in yellowtail muscle and this caused the degradation of MHC. The optimum pH and temperature of MBESP in yellowtail were 5.0 and 50–60°C, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The yellowtail Seriola quinqueradiata is one of the most important fish among cultured species in Japan. According to data from the Fisheries Agency of Japan, cultured yellowtail accounted for approximately 70% of total yellowtail production in 2006. In Japan, yellowtail meat is traditionally and popularly eaten raw as sashimi. However, variation in the quality of yellowtail raw meat has been recently observed during the summer time, and this is referred to as “burnt meat” [1].
“Burnt meat” is a term used by aquaculturists and commercial buyers to describe the white coloration of yellowtail or tuna muscle. It is pale, grainy and exudative, and is considered unsuitable for consumption as raw meat. Previous studies demonstrated that the burnt meat appearance is also observed in pork and meat of other fish species, and these researches suggested that the deterioration in the quality of burnt meat may be caused by high temperature in the environment, low ultimate muscle pH and slaughter methods [2–5]. In addition, myofibrillar proteins of the pale, soft and exudative (PSE) meat broke down with aging [6–8]. Konagaya and Konagaya [9] reported that approximately 60–90% of myofibrillar proteins were denatured in burnt red-meat fish. Mora et al. [1] investigated the characteristics of the burnt meat of cultured yellowtail and found that the ultimate decrease in muscle pH and the degradation of the myofibrillar proteins may lead to the burnt meat appearance in yellowtail.
In marine and freshwater fish, degradation of myofibrillar proteins is also the main cause of the modori phenomenon (thermal gel degradation of fish jelly products at around 55°C) during the manufacturing process [10, 11]. Several studies have shown that the different types of endogenous protease, such as cathepsins [12–16], aspartic acid proteases [17] or serine proteinases [18, 19], especially the myofibril-bound serine proteinases [20, 21], led to the degradation of myofibrillar proteins in fish meat.
In this study, the authors investigated the degradation of myofibrillar proteins, especially the degradation of the myosin heavy chain (MHC), of burnt meat of cultured yellowtail with different breeding water temperatures (17 and 29°C) and different slaughter methods. Furthermore, the protease that leads to the degradation of MHC was also investigated to clarify the relationship between the degradation of myofibrillar proteins and proteases in burnt meat of cultured yellowtail.
Materials and methods
Materials
Yellowtail Seriola quinqueradiata (average fork length 68.85 ± 4.50 cm, average weight 5.40 ± 0.60 kg) samples were obtained from a culture farm in Nagasaki Prefecture, Japan, during the summer and winter periods. Water temperatures at the sampling site in summer and in winter were 29 and 17°C, respectively. Fish kept at 17°C were used as the control during the assays.
Slaughter methods and sampling
Two slaughter methods, including spinal cord destruction (SCD) and suffocation in air (SA), were conducted following the procedure described by Mora et al. [1]. After slaughtering, the viscera of the fish were removed, and the heads and tails were cut off. Fish blocks were wrapped up in Kimtowels containing 10 mM sodium azide solution to avoid contamination by microorganisms, and then placed in plastic bags and stored at 30°C. Sampling was made at 0 (immediately after slaughtering), 2, 4, 6, 12 and 24 h of storage. Dorsal ordinary muscle samples were cut into 1-cm-thick slices for measurement of color and pH, and for analysis of the degradations of myofibrillar proteins.
Evaluation of meat quality
One-centimeter-thick slices of dorsal ordinary muscles (per sampling time) were used for color measurement. The color of sliced muscles was measured with a Minolta CR-200b color chromameter (Konica-Minolta, Tokyo, Japan). Color variations were obtained as L* (lightness), a* (red-green chromaticity) and b* (yellow-blue chromaticity) [22]. The measurement was carried out four times for each sample, and the values were averaged. In addition, 1-g samples of the ordinary muscle (per sampling time) were homogenized in 9 ml of distilled water. After homogenization, the pH value of the homogenate was immediately measured using a SevenEasy S20 pH meter (Mettler-Toledo International Inc., Tokyo, Japan).
Preparation of myofibrils from muscle samples
Each 50-mg muscle sample (0–24 h) was minced and homogenized with six volumes of ice-cold buffer (0.1 M KCl, 40 mM boric acid, 50 mM sodium borate, pH 7.0) using a Physcotron NS-50 homogenizer (Microtec Co., Ltd., Funabashi, Japan). The homogenization process was performed on ice, and the operation was repeated three times (30 s each time and an interval of 2 min) and centrifuged at 9,000 rpm at 4°C for 20 min. The supernatant was discarded, and the pellet was collected and resuspended in buffer. Myofibril pellets of cultured yellowtail were obtained after repeating the above procedure three times. Myofibrils were mixed with 1 ml of SDS buffer (20 mM Tris–HCl, pH 8.0, 8 M urea, 2% sodium dodecylsulfate, 0.02% bromphenol blue and 7.5% 2-mercaptoethanol) and heated in boiling water for 5 min. The protein concentration of myofibrils was determined by the method of Lowry et al. [23] after proper dilution. Bovine serum albumin was used as a standard.
Sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis
SDS-PAGE was performed under reducing conditions with 8, 8.5 or 5–15% gels according to the method of Laemmli [24]. After electrophoresis, protein bands were visually detected by staining with Coomassie Brilliant Blue R-250 (CBB).
Inhibition of myofibrillar protein degradation using different protease inhibitors
The inhibiting effects of different kinds of protease inhibitors on the degradation myofibrillar proteins were further investigated to identify active proteases. Myofibrils used were isolated from muscles of newly slaughtered SCD groups. The experiment was performed using 5 mg myofibril suspended in 50 mM acetic acid–sodium acetate buffer (pH 5.8, the pH values of yellowtail burnt meat) containing different types of protease inhibitors (Pefabloc SC, E-64, pepstatin A, EDTA, o-phenanthroline and phosphoramidon). The mixture was incubated at 30°C for 12 h, and effects of inhibitors on myofibrillar protein degradation were detected by SDS-PAGE using 8% polyacrylamide gels.
Effects of pH and temperature on the degradation of myofibrillar proteins
The effect of pH (from 1.5 to 9.0) on the degradation of myofibrillar protein was determined at 37°C by using 0.1 M incubation buffers: HCl–sodium acetate buffer (pH 1.5–3.0), acetic acid–sodium acetate buffer (pH 4.0–6.0) and Tris–HCl buffer (7.0–9.0). Myofibrils were isolated from the muscle of the newly slaughtered SCD 17°C group. Mixtures were incubated for 12 h, and the effect of pH on the degradation was analyzed by SDS-PAGE using 8.5% polyacrylamide gels. The effect of temperatures (from 20 to 70°C) on the degradation of myofibrillar protein, which was incubated for 12 h in 0.1 M acetic acid-sodium acetate buffer (pH 5.0), was analyzed by SDS-PAGE.
Results
Changes in muscle color
The color of yellowtail muscle after it was slaughtered was brilliant pink and was transparent in appearance, while the burnt meat muscle was white in color and turbid in appearance. Changes in lightness (L*) on yellowtail ordinary muscle from the two breeding temperatures and two slaughter methods during storage at 30°C are shown in Fig. 1. Muscles with lightness values of L* ≥ 55 [1] were evaluated as burnt meat. The L* value of the SA 29°C group was 60 within 2 h, indicating that the burnt meat phenomenon had progressed. In the SCD 29°C group, the burnt meat was also observed within 4 h, and the L* value was 59. In the case of the SA 17°C group, the L* value gradually rose post-mortem, and the burnt meat appeared at 4 h. In the SCD 17°C group, the L* value showed no significant change until 12 h, and the L* value was 55 after 24 h storage.
Changes in ultimate muscle pH
Changes in ultimate muscle pH of yellowtail ordinary muscle from the two breeding temperatures and two slaughter methods during storage at 30°C are shown in Fig. 2. In the SCD 29°C group, the pH value at 0 h storage was 6.4, which was higher than that of the other groups. In the SCD 29°C groups, pH values declined rapidly during the 6 h storage, and the pH value was lowest at 5.5, after which few changes were observed. In the SCD 17°C group, the initial pH value (0 h) was 6.3, which decreased to 6.0 after 2 h storage and remained constant at around 6.0 during the storage period. In the SA 29 and 17°C groups, pH values were 5.7 and 5.9 at 0 h storage, respectively. Moreover, pH values of both groups showed no significant changes during storage. These results suggested that pH was greatly affected by the high breeding temperature and slaughter method.
Degradation of myofibrillar proteins
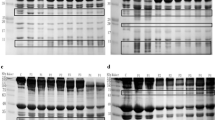
The change in the SDS-PAGE profile of myofibrillar proteins from two slaughter methods with storage time is shown in Fig. 3. For both groups, SDS-PAGE gels showed that MHC decreased with time during the 24 h storage. In the SCD 29°C group, increases in the 97-, 50-, 42- and 38-kDa bands were observed when the burnt meat appeared after 4 h. In the SA 29°C group, degradation of myofibrillar proteins during storage was faster than that of the SCD 29°C group. The 50-kDa band was found in newly slaughtered samples. With the appearance of burnt meat after 2 h, increases in the 97-, 42- and 38-kDa bands were found. In the 17°C groups, little degradation was observed during storage, and an increase of 50 kDa was found after 6 h storage in the SCD group and after 4 h in the SA group (data not shown). No degradation of actin was observed in any of the groups.
SDS-polyacrylamide gel electrophoresis (5–15% gels) of myofibrillar protein degradation on yellowtail ordinary muscle from two slaughter methods in summer (breeding temperature is 29°C) during storage at 30°C. Lanes M prestained standard marker; 0 non-storage control; 2–24 storage times. Arrowheads indicate the positions of myosin heavy chain (MHC) and actin. The blank arrows indicate the proteolytic products
Effect of protease inhibitors on the degradation of myofibrillar proteins
Different protease inhibitors were added to the myofibrils, and their suppressive effects on proteolytic activity are shown in Fig. 4. Compared to the 0 h group, the control showed decreased intensity of the protein band, completely corresponding to MHC (220 kDa). EDTA, an inhibitor of metalloproteases, completely suppressed the degradation of MHC in comparison to the control (Fig. 4a). Cysteine protease inhibitor E-64 and aspartic protease inhibitor pepstatin A both slightly inhibited the degradation of MHC. On the other hand, Pefabloc SC, a serine protease inhibitor, did not show any inhibitory effect on MHC degradation. In Fig. 4b, EDTA completely suppressed the degradation of MHC, and three other protease inhibitors showed no inhibiting effect. In Fig. 4c, the effect of three metalloprotease inhibitors on degradation of myofibrillar proteins is shown. Compared to the control, EDTA completely suppressed the degradation of MHC, while the metalloprotease inhibitors o-phenanthroline and phosphoramidon showed no inhibitory effect on MHC degradation. These results suggest that a myofibril-bound EDTA-sensitive protease (MBESP) exists in yellowtail muscle and may have caused the degradation of MHC.
SDS-polyacrylamide gel electrophoresis (8% gels) analysis of the inhibitory effect of protease inhibitors on degradation of myofibrillar proteins from yellowtail. Myofibrils were isolated from muscles of the SCD 29°C group (a) and 17°C group (b, c) just after slaughter. Myofibrils were dissolved in 50 mM acetic acid-sodium acetate buffer (pH 5.8) and different types of inhibitors. Lanes M, molecular-weight marker; (1), 0 h; (2) no inhibitor 12 h (control); (3) Pefabloc SC to the final concentration of 5 mM; (4) E-64 to 0.01 mM; (5) pepstatin A to 0.01 mM; (6) EDTA to 5 mM; (7) o-phenanthroline to 5 mM; (8) phosphoramidon to 100 μg/ml, respectively, incubated at 30°C for 12 h. Arrowheads indicate the positions of myosin heavy chain (MHC) and actin
Degradation of myofibrillar proteins at different pHs and temperatures
The effect of pH on degradation of myofibrillar proteins by MBESP from yellowtail is shown in Fig. 5a. Compared to the MHC band at 0 h, the original MHC bands were thinner at pH 3.9–6.9. The intensity degradation of MHC was observed at pH 5.0 in Fig. 5a and was inhibited by EDTA (data not shown). The effect of temperature on degradation of myofibrillar proteins by MBESP is shown in Fig. 5b. MHC was degraded to some degree at 20–70°C. At 50–60°C, the degradation of MHC was strong, but this was inhibited by EDTA. Degradation of MHC was also observed at 70°C when incubated without EDTA, but degradation was partially suppressed by EDTA.
SDS-polyacrylamide gel electrophoresis (8.5% gels) analysis of the effect of pH (a) and temperature (b) on degradation of myofibrillar proteins by myofibril-bound EDTA-sensitive protease (MBESP) from yellowtail. Myofibrils were isolated from the meat of the SCD 17°C group just after slaughter. a Myofibrils were incubated with 0.1 M buffer at different pHs for 12 h at 37°C. b Myofibrils were incubated with 0.1 M acetic acid–sodium acetate buffer (pH 5.0) at different temperatures for 12 h, incubation with EDTA (+) or without EDTA (−). Lanes M molecular-weight marker; 0 h: non-incubation. Arrowheads indicate the positions of myosin heavy chain (MHC) and actin
Discussion
In this study, the authors demonstrated that the appearance of burnt meat was accompanied by the degradation of MHC and changes in protein profile. Moreover, protease inhibitors E-64, pepstatin A, Pefabloc SC, o-phenanthroline and phosphoramidon all showed no significant inhibitory effect on the suppression of MHC degradation. In contrast, EDTA, an inhibitor of metalloproteases, completely suppressed the degradation of MHC.
Muscle color (L*) and ultimate muscle pH are useful as indicators for assessment of burnt meat [1]. When the fish was slaughtered and stored at 30°C, burnt meat (with muscle color parameter, L* ≥ 55) was observed after 2 h storage in the SA 29°C group, and after 4 h in SCD 29°C and SA 17°C groups. By contrast, the meat was normal in the SCD 17°C group until after 12 h storage (Fig. 1). These results imply that temperature and the slaughter method play an important role in the occurrence of burnt meat in yellowtail. This finding is consistent with the report by Mora et al. [1].
In this study, the muscle pH value ranged from 5.5 to 5.9 when the burnt meat appeared (Fig. 2). The pH values of the 29°C group were lower than those of the 17°C group. In the SCD groups, pH values dramatically decreased during the initial stage of storage. The lower muscle pH can lead to negative effects (such as poor liquid-holding capacity and a rapid degradation of the muscle proteins) on the quality of fish according to several reports [17, 25, 26]. During the culture of some fishes, intensive feeding increased the glycogen level in the muscle [17, 25, 26]. Post-mortem, high muscle temperature caused rapid glycolysis, which increased the lactic acid content and consequently caused the drop in pH [1, 27]. The phenomenon was similar to previous reports on burnt meat of tuna and pork [27–31].
The present result clearly showed a pH-dependent degradation of MHC in yellowtail burnt meat (Fig. 3). Yu and Lee [32] suggested that in bovine muscle, pH dictated the degradation of myofibrillar proteins in the post-mortem muscle. A previous study demonstrated that myosin has an isoelectric point at pH 5.5 [17], and proteins may be more susceptible to degradation at pH close to their isoelectric points [17, 33]. Therefore, this may also contribute to the increased degradation at the lower pH [17]. With the appearance of burnt meat, MHC was degraded, and some changes in 97-, 50-, 42- and 38-kDa bands of the myofibrils were observed. These protein bands may be the proteolytic products of myofibrils in yellowtail burnt meat. A similar phenomenon was also observed in PSE meat of pork [34].
Previous studies demonstrated that the degradation of MHC by endogenous protease, especially myofibril-bound serine proteinase (MBSP), is generally regarded as the main cause of the modori phenomenon [20]. When this study was conducted, it was expected that the degradation of MHC in cultured yellowtail burnt meat may also be caused by MBSP.
In order to identify which protease caused the degradation of MHC in yellowtail burnt meat, specific protease inhibitors, including Pefabloc SC (MBSP inhibitor), were added to the myofibrils, and their inhibiting effects on hydrolytic activity were investigated (Fig. 4). EDTA was found to completely suppress the degradation of MHC, while the other metalloprotease inhibitors o-phenanthroline and phosphoramidon showed no inhibitory effect on MHC degradation. These results suggest that MBESP might be associated with myofibrils of yellowtail muscle. In addition, E-64 and pepstatin A had weak inhibiting effects on MHC degradation, indicating that acid cysteine proteases (cathepsins B and L) and aspartic acid protease (cathepsin D) are possibly involved in MHC degradation during storage. In contrast, Pefabloc SC, an inhibitor for serine proteases, did not show any inhibitory effect on MHC degradation, suggesting that the MBSP may not exist in yellowtail muscles or the activity of MBSP may be weak.
Although MBSP were not obviously observed in yellowtail muscles, the existence of MBSP has been identified in some kinds of animals. For example, Hori et al. [35] purified chymotrypsin-type serine proteinases from the skeletal muscle of the mouse. Sangorrin et al. [36] partially purified a trypsin-type serine proteinase from the skeletal muscle of the mouse. Tshidino et al. [37] partially purified a MBSP from ostrich Struthio camelus skeletal muscle. MBSPs were also detected in several fishes, including carp Cyprinus carpio [10, 20], wanieso lizardfish Saurida wanieso [20], brushtooth lizardfish Saurida undosquamis [38] and crucian carp Carassius auratus [11].
In this study, we found that MBESP may exist in burnt meat of cultured yellowtail, and this may be involved in the degradation of MHC. In our previous studies on myofibril proteolysis, the degradation of MHC in pork, fowl and some fishes, such as red stingray and puffer fish, was inhibited by the EDTA (data not shown). This suggests that MBESP may also exist in some kinds of animals. Nevertheless, this warrants further investigation.
The degradation of myofibrils by MBESP at different pHs and temperatures was investigated on SDS-PAGE (Fig. 5). MBESP strongly degraded MHC at pH 5.0 and 50–60°C. Very little change in MHC at pH 6.9 indicated that the neutral proteases are not important for this breakdown. In carp Cyprinus carpio and wanieso lizardfish Saurida wanieso, the optimum pH and temperature of the MBSP were pH 7.0–8.0 and 55–60°C, respectively [20, 39]. Both MBSP and MBESP have optimum temperatures of around 50–60°C for degradation of MHC. MBSP had been reported to cleave MHC at neutral pH [10], while MBESP degraded MHC in acid in the present study. Therefore, the degradation of myofibrillar proteins in burnt meat of cultured yellowtail may be caused by MBESP.
To clarify the involvement between the degradation of myofibrillar proteins in cultured yellowtail burnt meat and MBESP, the authors are now trying to isolate this protease from myofibrils and then analyze its enzymatic characteristics.
References
Mora DA, Hamada Y, Okamoto A, Tateishi A, Tachibana K (2007) Characteristics of burnt meat in cultured yellowtail Seriola quinqueradiata. Fish Sci 73:651–659
Konagaya S, Konagaya T (1979) Acid denaturation of myofibrillar protein as the main cause of formation of “yake-niku”, a spontaneously done meat, in red meat fish. Nippon Suisan Gakkaishi 45:245
Cramer JL, Nakamura RM, Dizon AE, Ikehara WN (1981) Burnt tuna: conditions leading to rapid deterioration in the quality of raw tuna. Mar Fish Rev 43:12–16
Davie P, Sparksman RI (1986) Burnt tuna: an ultrastructural study of postmortem changes in muscle of yellowfin tuna (Thunnus albacores) caught on rod and reel and southern bluefin tuna (Thunnus maccoyii) caught on handline or longline. J Food Sci 51:1122–1128
Mishima T, Nonaka T, Okamoto A, Tsuchimoto M, Ishiya T, Tachibana K, Tsuchimoto M (2005) Influence of storage temperatures and killing procedures on post-mortem changes in the muscle of horse mackerel caught near Nagasaki Prefecture, Japan. Fish Sci 71:187–194
Wilson GGIII, Van Laack RLJM (1999) Sarcoplasmic proteins influence water-holding capacity of pork myofibrils. J Sci Food Agric 79:1939–1942
Wang H, Pato MD, Shand P (2005) Biochemical properties of natural actomyosin extracted from normal and pale, soft, and exudative pork loin after frozen storage. J Food Sci 30C:313–320
Van Laack RLJM, Liu CH, Smith MO, Loveday HD (2000) Characteristics of pale, soft, exudative broiler breast meat. Poultry Sci 79:1057–1061
Konagaya S, Konagaya T (1978) Denaturation at moderate temperatures of myofibrillar protein of red-meat fish: a possible cause of yake-niku. Bull Tokai Res Lab 96:67–74 (in Japanese with English abstract)
Cao MJ, Hara K, Osatomi K, Tachibana K, Izumi T, Ishihara T (1999) Myofibril-bound serine proteinase (MBP) and its degradation of myofibrillar proteins. J Food Sci 64:644–647
Cao MJ, Jiang XJ, Zhong HC, Zhang ZJ, Su WJ (2006) Degradation of myofibrillar proteins by a myofibril-bound serine proteinase in the skeletal muscle of crucian carp (Carasius auratus). Food Chem 94:7–13
Aranishi F, Ogata H, Hara K, Osatomi K, Ishihara T (1998) Susceptibility of opioid peptides and myofibrillar proteins to carp cathepsin L. J Agric Food Chem 46(2):388–392
Ogata H, Aranishi F, Hara K, Osatomi K, Ishihara T (1998) Proteolytic degradation of myofibrillar components by carp cathepsin L. J Sci Food Agric 76:499–504
An H, Weerasinghe V, Seymour TA, Morrissey MT (1994) Cathepsin degradation of Pacific whiting surimi proteins. J Food Sci 59(5):1013–1017
Nakamura S, Ogawa M, Saito M, Nakai S (1998) Application of polymannosylated cystatin to surimi from roe-herring to prevent gel weaking. FFBS Lett 427:252–254
Godiksen H, Morzel M, Hyldig G, Jessen F (2009) Contribution of cathepsins B, L and D to muscle protein profiles correlated with texture in rainbow trout (Oncorhynchus mykiss). Food Chem 113(4):889–896
Wang PA, Vang B, Pedersen AM, Martinez I, Olsen RL (2011) Post-mortem degradation of myosin heavy chain in intact fish muscle: Effect of pH and enzyme inhibitors. Food chem 124:1090–1095
Kinoshita M, Toyohara H, Shimizu Y (1990) Purification and properties of a novel latent proteinase showing myosin heavy chain degrading activity from threadfin-bream muscle. J Biochem 107:587–591
Yanagihara S, Nakaoka H, Hara K, Ishihara T (1991) Purification and characterization of serine proteinase from white croaker skeletal muscle. Nippon Suisan Gakkaishi 57:133–142
Cao MJ, Osatomi K, Hara K, Ishihara T (2000) Identification of a myofibril-bound serine proteinase (MBSP) in the skeletal muscle of Lizard fish Saurida wanieso which specifically cleaves the arginine site. Comp Biochem Physiol 125B:255–264
Du XL, Du CH, Liu GM, Wang XC, Hara K, Su WJ, Cao MJ (2010) Effect of a myofibril-bound serine proteinase on the deg of giant protein titin and nebulin. J Food Biochem 34:581–594
Hunt RWG (1970) The specification of colour appearance. 1. Concepts and terms. Color Res Appl 2:55–68
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:256–275
Laemmli UK (1970) Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nature 277(5259):680–685
Ang JF, Haard NF (1985) Chemical composition and postmortem changes in soft textured muscle from intensely feeding Atlantic cod (Gadus morhua L.). J Food Biochem 9(1):49–64
Ofstad R, Egelandsdal B, Kidman S, Myklebust R, Olsen RL, Hermansson AM (1996) Liquid loss as effected by post-mortem ultrastructural changes in fish muscle: Cod (Gadus morhua L.) and salmon (Salmo salar). J Sci Food Agric 71(3):301–312
Ochiai Y (2010) Changes in quality and denaturation of sarcoplasmic protein components in the burnt meat of bluefin tuna Thunnus thynnus orientalis. Nippon Suisan Gakkaishi 76(4):695–704 (in Japanese with English abstract)
Oka H, Kazuhito O, Ninomiya J (1990) Changes in texture during cold storage of cultured yellowtail meat prepared by different killing methods. Nippon Suisan Gakkaishi 56:1673–1678 (in Japanese with English abstract)
Fischer K, Augustini C (1977) Stadien der postmortalen glykogenolyse bie unterschiedlichen pH1-Werten in scheweinefleich. Die Fleishwirtschaft 6:1191–1194 (in German)
McCurdy RD, Barbut S, Quinton M (1996) Seasonal effect on pale soft exudative (PSE) occurrence in young turkey breast meat. Food Res Int 29:363–366
Warriss PD, Brown SN, Pasciak P (2006) The colour of the adductor muscle as a predictor of pork quality in the loin. Meat Sci 73:565–569
Yu LP, Lee YB (1986) Effects of postmortem pH and temperature on bovine muscle structure and meat tenderness. J Food Sci 51:774–780
Dice JF, Goldberg AL (1975) Relationship between in vivo degradative rates and isoelectric points of proteins. Proc Natl Acad Sci USA 72(10):3893–3897
Laville E, Sayd T, Sante′-Lhoutellier V, Morzel M, Labas R, Franck M, Chambon C, Monin M (2005) Characterisation of PSE zones in semimembranosus pig muscle. Meat Sci 70:167–172
Hori S, Ohtani S, Hori C, Nokihara K (1998) Purification and characterization of myonase from X-chromosome linked muscular dystrophic mouse skeletal muscle. J Biochem (Tokyo) 123:650–658
Sangorrin MP, Martone CB, Sanchez JJ (2002) Myofibril-bound serine protease and its endogeneous inhibitor in muscle: extraction, partial characterization and effect on myofibrils. Comp Biochem Physiol 131B:713–723
Tshidino SC, Krause J, Adebiyi AP, Muramoto K, Naude RJ (2009) Purification and partial characterization of a myofibril-bound serine protease from ostrich skeletal muscle. Comp Biochem Physiol 154B:229–234
Ohkubo M, Miyagawa K, Osatomi K, Hara K, Nozaki Y, Ishihara T (2004) A novel serine protease complexed with α2-macroglobulin from skeletal muscle of lizard fish (Saurida undosqyamis). Comp Biochem Physiol 139B:637–647
Osatomi K, Sasai H, Cao MJ, Hara K, Ishihara T (1997) Purification and characterization of myofibril-bound serine proteinase from carp Cyprinus carpio ordinary muscle. Comp Biochem Physiol 116B:183–190
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Liang, X., Yoshida, A., Osatomi, K. et al. Degradation of myofibrils in cultured yellowtail Seriola quinqueradiata burnt meat: effects of a myofibril-bound EDTA-sensitive protease. Fish Sci 78, 147–153 (2012). https://doi.org/10.1007/s12562-011-0429-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-011-0429-5