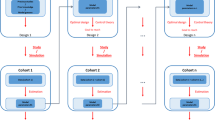
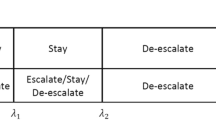
Abstract
Precision oncology has demonstrated the potential of drug combinations in effectively enhancing anti-tumor efficiency and controlling disease progression. Nonetheless, dose optimization in early-phase drug combination trials presents various challenges and is considerably more complex than single-agent dose optimization. To address this, we propose a surface-free design for exploring the optimal doses of combination therapy within the phase I–II framework. Rather than relying on parametric models to define the shape of toxicity and efficacy surfaces, our approach centers on characterizing dose-toxicity and dose-efficacy relationships between adjacent dose combinations using surface-free models. The proposed design encompasses a run-in phase, facilitating a swift exploration of the dose space, followed by a main phase where the dose-finding rule relies on the proposed surface-free model. Through extensive simulation studies, we have thoroughly examined the operating characteristics of this innovative design. The results demonstrate that our method exhibits desirable operating characteristics across a wide range of dose-toxicity and dose-efficacy relationships.



Similar content being viewed by others
References
Yan F, Thall PF, Lu KH, Gilbert MR, Yuan Y (2018) Phase I-II clinical trial design: a state-of-the-art paradigm for dose finding. Ann Oncol 29(3), 694–699 https://doi.org/10.1093/annonc/mdx795 . APOBEC mutagenesis in HIV and cancer evolution
Braun TM (2002) The bivariate continual reassessment method: extending the crm to phase I trials of two competing outcomes. Controlled Clin Trials 23(3):240–256. https://doi.org/10.1016/S0197-2456(01)00205-7
Thall PF, Cook JD (2004) Dose-finding based on efficacy-toxicity trade-offs. Biometrics 60(3):684–693 https://doi.org/10.1111/j.0006-341X.2004.00218.xhttps://onlinelibrary.wiley.com/doi/pdf/10.1111/j.0006-341X.2004.00218.x
Yin G, Li Y, Ji Y (2006) Bayesian dose-finding in phase I/II clinical trials using toxicity and efficacy odds ratios. Biometrics 62(3):777–787. https://doi.org/10.1111/j.1541-0420.2006.00534.x
Wages NA, Tait C (2015) Seamless phase I/II adaptive design for oncology trials of molecularly targeted agents. J Biopharma Stat 25(5):903–920. https://doi.org/10.1080/10543406.2014.920873. (( PMID: 24904956))
Zang Y, Lee JJ (2017) A robust two-stage design identifying the optimal biological dose for phase I/II clinical trials. Stat Med 36(1):27–42. https://doi.org/10.1002/sim.7082
Riviere M-K, Yuan Y, Jourdan J-H, Dubois F, Zohar S (2018) Phase I/II dose-finding design for molecularly targeted agent: Plateau determination using adaptive randomization. Stat Meth Med Res 27(2):466–479. https://doi.org/10.1177/0962280216631763
Takeda K, Taguri M, Morita S (2018) BOIN-ET: Bayesian optimal interval design for dose finding based on both efficacy and toxicity outcomes. Pharma Stat 17(4):383–395. https://doi.org/10.1002/pst.1864
Zhou Y, Lee JJ, Yuan Y (2019) A utility-based Bayesian optimal interval (U-BOIN) phase I/II design to identify the optimal biological dose for targeted and immune therapies. Stat Med 38(28):5299–5316. https://doi.org/10.1002/sim.8361
Muenz DG, Taylor JMG, Braun TM (2019) Phase I-II trial design for biologic agents using conditional auto-regressive models for toxicity and efficacy. J Royal Stat Soc Series C (Applied Statistics) 68(2):331–345. https://doi.org/10.1111/rssc.12314
Li P, Liu R, Lin J, Ji Y (2020) TEPI-2 and UBI: designs for optimal immuno-oncology and cell therapy dose finding with toxicity and efficacy. J Biopharma Stat 30(6):979–992. https://doi.org/10.1080/10543406.2020.1814802. (PMID: 32951518)
Lin R, Zhou Y, Yan F, Li D, Yuan Y (2020) BOIN12: Bayesian optimal interval phase I/II trial design for utility-based dose finding in immunotherapy and targeted therapies. JCO Precision Oncol 4:1393–1402. https://doi.org/10.1200/PO.20.00257
Lu DY (2015) 6 - drug combinations. In: Lu, D.-Y. (ed.) Personalized Cancer Chemotherapy, pp. 37–41. Woodhead Publishing, Oxford. https://doi.org/10.1016/B978-0-08-100346-6.00006-6 . https://www.sciencedirect.com/science/article/pii/B9780081003466000066
Wages NA, Chiuzan C, Panageas KS (2018) Design considerations for early-phase clinical trials of immune-oncology agents. J ImmunoTherapy Cancer. https://doi.org/10.1186/s40425-018-0389-8
Thall PF, Millikan RE, Mueller P, Lee S-J (2003) Dose-finding with two agents in phase I oncology trials. Biometrics 59(3):487–496. https://doi.org/10.1111/1541-0420.00058
Yin G, Yuan Y (2009) Bayesian dose finding in oncology for drug combinations by copula regression. J Royal Stat Soc Series C (Applied Statistics) 58(2):211–224. https://doi.org/10.1111/j.1467-9876.2009.00649.x
Wages NA, Conaway MR, O’Quigley J (2011) Continual reassessment method for partial ordering. Biometrics 67(4):1555–1563. https://doi.org/10.1111/j.1541-0420.2011.01560.x
O’Quigley J, Pepe M, Fisher L (1990) Continual reassessment method: a practical design for phase 1 clinical trials in cancer. Biometrics, 33–48
Mozgunov P, Gasparini M, Jaki T (2020) A surface-free design for phase I dual-agent combination trials. Stat Meth Med Res 29(10):3093–3109. https://doi.org/10.1177/0962280220919450. (PMID: 32338145)
Braun TM, Wang S (2010) A hierarchical Bayesian design for phase I trials of novel combinations of cancer therapeutic agents. Biometrics 66(3):805–812. https://doi.org/10.1111/j.1541-0420.2009.01363.x
Wages NA, Conaway MR, O’Quigley J (2011) Dose-finding design for multi-drug combinations. Clin trials (London, England) 8(4):380–389. https://doi.org/10.1177/1740774511408748
Riviere M-K, Yuan Y, Dubois F, Zohar S (2014) A Bayesian dose-finding design for drug combination clinical trials based on the logistic model. Pharma Stat 13(4):247–257. https://doi.org/10.1002/pst.1621
Sun Z, Braun TM (2015) A two-dimensional biased coin design for dual-agent dose-finding trials. Clin Trials 12(6):596–607. https://doi.org/10.1177/1740774515592404. (PMID: 26163309)
Mu R, Xu J (2017) A new Bayesian dose-finding design for drug combination trials. Stat Biopharma Res 9(4):384–389. https://doi.org/10.1080/19466315.2017.1388834
Lin R, Yin G (2017) Bayesian optimal interval design for dose finding in drug combination trials. Stat Meth Med Res 26(5):2155–2167. https://doi.org/10.1177/0962280215594494. (PMID: 26178591)
Clertant M, Wages NA, O’Quigley J (2022) Semiparametric dose finding methods for partially ordered drug combinations. Statistica Sinica. https://doi.org/10.5705/ss.202020.0248
Huang X, Biswas S, Oki Y, Issa J-P, Berry DA (2007) A parallel phase I/II clinical trial design for combination therapies. Biometrics 63(2):429–436
Yuan Y, Yin G (2011) Bayesian phase I/II adaptively randomized oncology trials with combined drugs. Ann Appl Stat 5(2A):924–942
Cai C, Yuan Y, Ji Y (2014) A Bayesian dose finding design for oncology clinical trials of combinational biological agents. J Royal Stat Soc Series C (Applied Statistics) 63(1): 159–173
Guo B, Li Y (2015) Bayesian dose-finding designs for combination of molecularly targeted agents assuming partial stochastic ordering. Stat Med 34(5):859–875. https://doi.org/10.1002/sim.6376
Wages NA, Conaway MR (2014) Phase I/II adaptive design for drug combination oncology trials. Stat Med 33(12):1990–2003. https://doi.org/10.1002/sim.6097
Shimamura F, Hamada C, Matsui S, Hirakawa A (2018) Two-stage approach based on zone and dose findings for two-agent combination phase I/II trials. J Biopharma Stat 28(6):1025–1037. https://doi.org/10.1080/10543406.2018.1434190. (PMID: 29420127)
Yada S, Hamada C (2018) A Bayesian hierarchal modeling approach to shortening phase I/II trials of anticancer drug combinations. Pharma Stat 17(6):750–760. https://doi.org/10.1002/pst.1895
Portell CA, Wages NA, Kahl BS, Budde LE, Chen RW, Cohen JB, Varhegyi NE, Petroni GR, Williams ME (2022) Dose-finding study of ibrutinib and venetoclax in relapsed or refractory mantle cell lymphoma. Blood Adv 6(5):1490–1498. https://doi.org/10.1182/bloodadvances.2021005357
Iasonos A, Wages NA, Conaway MR, Cheung K, Yuan Y, O’Quigley J (2016) Dimension of model parameter space and operating characteristics in adaptive dose-finding studies. Stat Med 35(21):3760–3775
O’Quigley J, Conaway M (2010) Continual reassessment and related dose-finding designs. Stat Sci Rev J Inst Math Stat 25(2):202
Yuan Y, Lin R, Lee JJ (2022) Model-assisted Bayesian Designs for Dose Finding and Optimization: Methods and Applications. CRC Press, ???
Yuan Y, Lin R, Li D, Nie L, Warren KE (2018) Time-to-event Bayesian optimal interval design to accelerate phase I trials. Clin Cancer Res 24(20):4921–4930. https://doi.org/10.1158/1078-0432.CCR-18-0246
Lin R, Yuan Y (2019) Time-to-event model-assisted designs for dose-finding trials with delayed toxicity. Biostatistics 21(4):807–824. https://doi.org/10.1093/biostatistics/kxz007
Cheung YK, Chappell R (2000) Sequential designs for phase I clinical trials with late-onset toxicities. Biometrics 56(4):1177–1182. https://doi.org/10.1111/j.0006-341X.2000.01177.x
Morita S, Thall PF, Müller P (2008) Determining the effective sample size of a parametric prior. Biometrics 64(2):595–602
Zheng H, Hampson LV (2020) A Bayesian decision-theoretic approach to incorporate preclinical information into phase i oncology trials. Biometrical J 62(6):1408–1427
Petit C, Samson A, Morita S, Ursino M, Guedj J, Jullien V, Comets E, Zohar S (2018) Unified approach for extrapolation and bridging of adult information in early-phase dose-finding paediatric studies. Stat Meth Med Res 27(6):1860–1877
Lin R, Shi H, Yin G, Thall PF, Yuan Y, Flowers CR (2022) Bayesian hierarchical random-effects meta-analysis and design of phase i clinical trials. Ann Appl Stat 16(4):2481
Acknowledgements
We would like to thank the Editor, the Associate Editor, and the two reviewers for their valuable comments and suggestions, which greatly contributed to enhancing the quality of the article. Lin is partially supported by grants from the National Cancer Institute (5P30CA016672, 5P50CA221703, and 1R01CA261978). Wages is partially supported by grants from the National Cancer Institute (R01CA247932).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no Conflict of interest to declare that are relevant to the content of this article.
Appendix: Extending the Proposed Method to Include Three-Dose Combinations
Appendix: Extending the Proposed Method to Include Three-Dose Combinations
The proposed design can be straightforwardly generalized to the combination of three or more drugs. This appendix elucidates the models and dose optimization steps when three drugs are involved. Regarding the toxicity model, we assume \(\theta ^T=1-p_{111}\). Following the “equal-jump” assumption, the parameterization of \(\theta _j^T\), \(\tau _k^T\), and \(\nu _m^T\) is as follows:
The DLT rate of dose combination (j, k, m) takes the form
The efficacy probability of dose combination (j, k, m) can be derived similarly. Let \(\theta ^E=\textrm{logit}(q_{111})\). Following the “equal-jump” assumption, \(\theta _j^E\),\(\tau _k^E\), and \(\nu _m^E\) are parameterized as follows:
The MTDC for each specific axis, \(j_{km}^*\), \(k_{jm}^*\), \(m_{jk}^*\), can be elicited by extending (7) of the manuscript:
\({\mathcal {S}}\) is updated as:
-
(A)
If \(j_{km}^*>j\), \(k_{jm}^*>k\), and \(m_{jk}^*>m\), \({\mathcal{S}} = {\{} (j-1,k-1,m), (j-1,k,m-1), (j,k-1,m-1), (j-1,k,m), (j,k-1,m), (j,k,m-1), (j,k,m), (j+1,k,m), (j,k+1,m), (j,k,m+1), (j+1,k+1,m), (j+1,k,m+1), (j,k+1,m+1){\}}\);
-
(B)
If \(j_{km}^*=j\), \(k_{jm}^*>k\), and \(m_{jk}^*>m\), \({\mathcal {S}}=\{(j-1,k-1,m),\ (j-1,k,m-1),\ (j,k-1,m-1),\ (j-1,k,m),\ (j,k-1,m),\ (j,k,m-1),\ (j,k,m),\ (j,k+1,m),\ (j,k,m+1),\ (j,k+1,m+1)\}\);
-
(C)
If \(j_{km}^*>j\), \(k_{jm}^*=k\), and \(m_{jk}^*>m\), \({\mathcal {S}}=\{(j-1,k-1,m),\ (j-1,k,m-1),\ (j,k-1,m-1),\ (j-1,k,m),\ (j,k-1,m),\ (j,k,m-1),\ (j,k,m),\ (j+1,k,m),\ (j,k,m+1),\ (j+1,k,m+1)\}\);
-
(D)
If \(j_{km}^*>j\), \(k_{jm}^*>k\), and \(m_{jk}^*=m\), \({\mathcal {S}}=\{(j-1,k-1,m),\ (j-1,k,m-1),\ (j,k-1,m-1),\ (j-1,k,m),\ (j,k-1,m),\ (j,k,m-1),\ (j,k,m),\ (j+1,k,m),\ (j,k+1,m),\ (j+1,k+1,m)\}\);
-
(E)
If \(j_{km}^*=j\), \(k_{jm}^*=k\), and \(m_{jk}^*>m\), \({\mathcal {S}}=\{(j-1,k-1,m),\ (j-1,k,m-1),\ (j,k-1,m-1),\ (j-1,k,m),\ (j,k-1,m),\ (j,k,m-1),\ (j,k,m),\ (j,k,m+1)\}\);
-
(F)
If \(j_{km}^*=j\), \(k_{jm}^*>k\), and \(m_{jk}^*=m\), \({\mathcal {S}}=\{(j-1,k-1,m),\ (j-1,k,m-1),\ (j,k-1,m-1),\ (j-1,k,m),\ (j,k-1,m),\ (j,k,m-1),\ (j,k,m),\ (j,k+1,m)\}\);
-
(G)
If \(j_{km}^*>j\), \(k_{jm}^*=k\), and \(m_{jk}^*=m\), \({\mathcal {S}}=\{(j-1,k-1,m),\ (j-1,k,m-1),\ (j,k-1,m-1),\ (j-1,k,m),\ (j,k-1,m),\ (j,k,m-1),\ (j,k,m),\ (j+1,k,m)\}\);
-
(H)
If we have three \("="\), or two \("="\) and one \("<"\), or one \("="\) and two \("<"\), \({\mathcal {S}}=\{(j-1,k-1,m),\ (j-1,k,m-1),\ (j,k-1,m-1),\ (j-1,k,m),\ (j,k-1,m),\ (j,k,m-1),\ (j,k,m)\}\);
-
(I)
If \(j_{km}^*<j\), \(k_{jm}^*<k\), and \(m_{jk}^*<m\), \({\mathcal {S}}=\{(j-1,k-1,m),\ (j-1,k,m-1),\ (j,k-1,m-1),\ (j-1,k,m),\ (j,k-1,m),\ (j,k,m-1)\}\).
As per the definition of \({\mathcal {S}}\) provided earlier, the dose exploration of three drugs can be seamlessly integrated into the proposed dose-finding algorithm.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, J., Wages, N.A. & Lin, R. SFU: Surface-Free Utility-Based Design for Dose Optimization in Cancer Drug Combination Trials. Stat Biosci (2024). https://doi.org/10.1007/s12561-024-09424-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12561-024-09424-x