Abstract
Aquatic habitats provide a bridge for influenza transmission among wild and domestic species. However, water sources pose highly variable physicochemical and ecological characteristics that affect avian influenza virus (AIV) stability. Therefore, the risk of survival or transmissibility of AIV in the environment is quite variable and has been understudied. In this study, we determine the risk of waterborne transmission and environmental persistence of AIV in a wild/domestic bird interface in the Central Mexico plateau (North America) during the winter season using a multi-criteria decision analysis (MCDA). A total of 13 eco-epidemiological factors were selected from public-access databases to develop the risk assessment. The MCDA showed that the Atarasquillo wetland presents a higher persistence risk in January. Likewise, most of the backyard poultry farms at this wild-domestic interface present a high persistence risk (50%). Our results suggest that drinking water may represent a more enabling environment for AIV persistence in contrast with wastewater. Moreover, almost all backyard poultry farms evidence a moderate or high risk of waterborne transmission especially farms close to water bodies. The wildlife/domestic bird interface on the Atarasquillo wetland holds eco-epidemiological factors such as the presence of farms in flood-prone areas, the poultry access to outdoor water, and the use of drinking-water troughs among multiple animal species that may enhance waterborne transmission of AIV. These findings highlight the relevance of understanding the influence of multiple factors on AIV ecology for early intervention and long-term control strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environment plays a key role in viral transmission among infected and susceptible hosts (Keeler et al., 2014). Infected animals can introduce avian influenza viruses (AIVs) into aquatic habitats through fecal contamination and tracheal shedding (Nielsen et al., 2013). The AIVs contaminate open water bodies and become a source of infection or reinfection among wild and domestic avian populations (Keeler et al., 2012; Zhang et al., 2014).
Migratory waterfowl stopovers in proximity to backyard farms facilitate the emergence of new viral strains and enhance cross-species transmission (Müller-Theissen et al., 2022; Zhang et al., 2014). Multiple viral subtypes have been isolated from water mostly obtained in areas where farms overlap with wetlands (Das Gupta et al., 2022; Karasin et al., 2000; Mateus-Anzola et al., 2021). Therefore, household and free-range poultry on spatiotemporal coincidence with wild birds pose a high risk for influenza outbreaks (Hassan et al., 2020).
Environmental surveillance and laboratory-based studies have evidenced that IAVs may remain viable for extended periods outside of a biotic reservoir mainly in cold environmental conditions (Keeler et al., 2014; Ramey et al., 2022). Viral survival and stability depend on wild bird habitats’ physicochemical and ecological characteristics (Dalziel et al., 2016; Keeler et al., 2014; Tran et al., 2010). Likewise, viral transmission relies on farm epidemiological factors (Huang et al., 2016). However, there is limited data related to the influence of eco-epidemiological factors in the transmission and persistence of AIVs in environments shared between multiple species (Stallknecht et al., 2010). This study aims to fill some of those knowledge gaps by determining the risk of waterborne transmission and environmental persistence of AIVs at the wild/domestic bird interface using a multi-criteria decision analysis (MCDA). We illustrate our approach using data collected in Mexico during the winter season 2019–2020.
Materials and Methods
Study Area
The study area comprises one of the Lerma marshes, the Atarasquillo wetland, located in the Municipality of Lerma de Villada, State of Mexico, North America (19°13′–19°26′N, 99°22′–99°34′W). This Flora and Fauna Protection Area hosts great biological diversity, including endemic and threatened species such as the Mexican duck (Anas diazi) and twelve migratory wild bird species from North America (SEMARNAT-CONANP, 2018). Therefore, the Atarasquillo wetland is considered an ecosystem with high local and regional relevance for wild bird conservation in Mexico (Zepeda et al., 2014).
The Atarasquillo wetland represents a priority hydrological region based on productive and sociocultural activities. The region’s economy comprises artisanal fisheries, traditional hunting of waterfowl, farming, and grazing (Zepeda-Gómez et al., 2012). This Important Bird and Biodiversity Area (IBA) is surrounded by agricultural and livestock production systems mainly backyard poultry and pig farms with low bio-security measures that facilitate interspecies transmission (Gaytan-Cruz et al., 2020; Mateus-Anzola et al., 2020).
Identification of Factors
Factors that influence the transmission and environmental persistence of AIVs were identified by a review of scientific literature. Three databases: Web of Science, PubMed, and Science Direct were searched for articles focused on the waterborne transmission of AIVs among wild birds and poultry, as well as the viral persistence in water. We used the search terms ((((influenza OR influenzavirus)) AND ((persistence OR survival OR stability OR viability OR inactivation OR tenacity OR survivability OR transmission OR infectivity OR infection OR infective OR infect)) AND ((surface water OR natural water OR wetland OR waterway OR watershed OR environmental water OR drinking water OR sewage OR wastewater)) AND ((factor)))). Studies in distilled water, peptone water, as well as human influenza viruses were excluded. Each factor was further evaluated through literature focusing on research articles with quantitative data and statistical significance on previous studies. A total of 13 eco-epidemiological factors were selected as inputs to develop risk assessments in a wild/domestic animal interface on the Atarasquillo wetland during the winter season (Table 1).
Data Collection
The eco-epidemiological variables were collected from the Atarasquillo wetland and 14 backyard poultry farms during the winter season 2019–2020 using a convenience sampling method. Four sampling sites were considered within the Atarasquillo wetland: three sites where hunting duck activities were practiced, and one site where wild duck plucking was carried out on the shore of the wetland close to human settlements, crops, and poultry backyard farms (Fig. 1). The drinking water, wastewater, drainage ditch, and artificial pond were considered as sampling locations in the backyard poultry production systems.
Water temperature (°C) (HANNA, HI-98127 pocket meter) and water pH (HANNA, HI-98127 pH meter) were measured on-site twice in each sampling site. Water electrical conductivity (µS/cm), water ammonia concentration-NH3 (mg/L), and water salinity (psu) were measured in 500 ml water samples using a YSI 6600 Multi Parameter V2 Sonde. Farms were geo-located using a global positioning system (GPS) device and distances to the Atarasquillo wetland centroid (m) were computed using ArcGis 10.8 (CONANP, 2022; INEGI, 2022). Flood/waterlogged areas were identified according to soil susceptibility to flooding (i.e., vertisol and phaeozem were considered flooded soils) (Barragán & Figueroa, 2014). The soil types were explored with ArcGis 10.8 (INEGI, 2015; INIFAP-CONABIO, 2008). Data related to aquaculture farming, poultry outdoor access, drinking water source and quality, as well as disposal of dead animals and wastage were obtained by cross-sectional surveys.
Weights Attribution for MCDA
Risk of environmental transmission and persistence of AIVs was determined by an MCDA approach similar to the one described by Zhao et al. (2022). The eco-epidemiological factors were considered as criteria and were weighted by the Mean Weight Method (Ezell et al., 2021; Odu, 2019), using the following equation:
where Wj is the criteria weight and n is the number of criteria.
The persistence MCDA model included five criteria (Wj = 0.2), while the transmission MCDA model accounted eight criteria (Wj = 0.125). The criteria were categorized in classes based on reference values. A weight from 0 to 3 was assigned to each class according to their suitability for AIV transmission or persistence in water (3 = high, 2 = mid, 1 = low, and 0 = none), where a higher value meant a higher likelihood of waterborne transmission or environmental persistence. The class weights (Wi) were normalized following the formula:
The final weight was calculated by multiplying the normalized class weight with the criteria weight. The final score was the sum of the final weights obtained (Tables 2 and 3).
Waterborne Transmission and Environmental Persistence Risk
The final scores from the MCDA were classified into five risk categories: very low, low, moderate, high, and very high risk (Stenkamp-Strahm et al., 2020). The threshold was determined by dividing the maximum final score (1.00) into five levels (Table 4).
For example, if we have a sample with the following values: C1: 21.0 °C (temperature), C2: 0.6 ppt (salinity), C3: 7.6 (pH), C4: 867.5 µS/cm (electrical conductivity), and C5: 0.4 mg/L (ammonia), the sample would be a moderate risk according to the MCDA model (Table 5).
Model Validation
A sensitivity analysis was performed to evaluate the stability of the outcomes under the uncertainty of the input variables. Although low pathogenic avian influenza viruses (LPAI) have been previously detected in resident and migratory wild bird species in the study area, no AIV has been reported in environmental samples of this wildlife/domestic bird interface (Gaytan-Cruz et al., 2020; Mateus-Anzola et al., 2020). Therefore, a one-at-a-time (OAT) method was conducted by varying one criterion at a time (Delgado & Sendra, 2004; Pianosi et al., 2016). The weight of one factor varied from 0 to 100% while the weights of the other criteria were adjusted to maintain the same percentage (e.g., an input weight of 60% on the variable factor represents weights of 10% to each of the other four criteria to sum 100%). Heat maps were used to represent the sensitivity analysis of the environmental persistence MCDAs (Online Resource 1 and 2) and the transmission persistence MCDA (Online Resource 3).
Results
Environmental Persistence Risk
The average water temperature, salinity, pH, electrical conductivity, and ammonia on the Atarasquillo wetland and the water of backyard poultry farms during the winter season 2019–2020 is shown in Table 6. Physicochemical characteristics could not be obtained on farms F13 and F14.
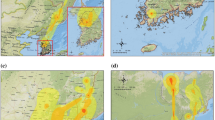
A high risk of environmental persistence was evidenced in December, February, and March, while a very high risk was evidenced in January, mainly between mid-January and early February on the Atarasquillo wetland (Fig. 2).
In relation to backyard poultry farms, a moderate (27.27%), high (45.46%), and very high (27.27%) persistence risk was evidenced in drinking water. A moderate (33.33%) and high (66.67%) risk was presented in the drainage ditch. A low (50%), moderate (25%), and high (25%) risk was evidenced in wastewater, meanwhile, a high risk (100%) was observed in all the artificial ponds within the farms. Most of the farms presented a high persistence risk (50%) followed by a moderate risk (25%). Only two farms evidenced a low persistence risk (10%). None of the farms had a very low persistence risk (Fig. 3).
Environmental Transmission Risk
Most of the backyard poultry farms were in flood-prone areas (71.42%) and almost 43% were located less than 2000 m from the Atarasquillo wetland’s centroid. Half of the poultry animals had access to outdoor water mainly to drainage ditches and artificial ponds. None of the animals have access to the Atarasquillo wetland. More than half of poultry (64.29%) share water troughs with another species and half of drinking water-throughs were dirty and contaminated with feces and feathers. Only three farms (21.42%) reported the disposal of poultry feces or eggs in/near water sources. None of the farmers mentioned the use of surface water as poultry drinking water.
Almost all the backyard poultry farms had a moderate or high risk of waterborne transmission (85.72%), meanwhile, low risk was evidenced only in 14.28% of them. The backyard poultry systems with lower risk were located away from the Atarasquillo wetland (>4.3 km) (Fig. 4).
Model Validation
The persistence risk on the Atarasquillo wetland was the same in the OAT analysis, in which a higher risk score was observed during January on the heat map. Likewise, the persistence risk and the transmission risk on backyard poultry farms were mostly comparable in the OAT analysis. A higher persistence risk score was evidenced in the drinking water, meanwhile, a lower risk score was evidenced in the wastewater on the heat map. Concerning the transmission risk, a higher risk was observed in six of the backyard poultry farms closest to the Atarasquillo wetland (F1 to F6) on the heat map (Online Resource 1, 2, and 3).
Discussion
This study describes an MCDA approach to determine the risk of environmental persistence and waterborne transmission of AIV in a wild/domestic bird interface in central Mexico. The AIVs spread within wild waterbird populations may lead to viral contamination of natural habitats (Ahrens et al., 2022). During the winter season, a high density of migrating Anseriformes cohabit with resident species in the Atarasquillo wetland (Gaytan-Cruz et al., 2020; SEMARNAT-CONANP, 2018). One of the most remarkable results is that the coldest month evidences a higher persistence risk of AIV. Previous research has found a strong effect of temperature on environmental viability (Dalziel et al., 2016; Martin et al., 2018). Virus may remain infective for a few days at >20 °C, a few weeks at 10 °C, and for months at <0 °C in surface water (Nazir et al., 2010). Therefore, freshwater habitats could be a year-to-year reservoir of viruses to infect bird populations mainly in winter (Lang et al., 2008; Ramey et al., 2022).
Water sources represent a crucial environment in which infectious AIVs may reside outside of a biotic reservoir (Ramey et al., 2022). Recently, some mass mortality events in free-living mammal species such as the harbor seal are likely associated to environmental transmission of HPAI H5N1. Likewise, global HPAI outbreaks in poultry are possibly linked to indirect contact with wild birds (European Food Safety Authority et al., 2023). In our study, we did not attempt to record contact between household animals and wild birds. However, almost all the backyard poultry farms close to the Atarasquillo wetland evidenced a higher transmission risk. This outcome is in line with Si et al. (2013), who reported the occurrence of outbreaks mostly in areas where the location of farms or animal trade areas overlap with habitats for wild birds. Therefore, animal populations close to wetlands pose a high risk of influenza outbreaks (Hassan et al., 2020).
Shallow water bodies represent an AIV transmission medium for aquatic wild birds. Fecal matter, plumage, and oropharyngeal excretions with viral particles potentiate viral transmission efficacy in surface waters (Ahrens et al., 2022). In our study, artificial ponds within backyard poultry farms and channels of water evidenced a high risk of AIV persistence. Previous research has reported that a low viral titer suspended in the surface water is sufficient to start and set off an infection in wild ducks within a few days, mainly in a low volume of water. Small water bodies can hold moderate to high viral RNA loads for a long period due to a lower diluting effect on the virus available for infection compared to large water bodies (Ahrens et al., 2022).
A limited volume of accessible water may provide high viral titers and a long course of infection (Ahrens et al., 2022). According to Leung et al. (2007) poultry drinking water can provide higher isolation rates of the influenza virus than fecal droppings. Likewise, drinking water troughs may contain a great AIV subtype diversity (Mateus-Anzola et al., 2021). Interestingly, in our study, a very high-risk persistence score was found in some poultry farms’ drinking water. Experimental laboratory studies have reported AIV survivability of 8–48 h in drinking water troughs, as well as a viral concentrating effect. Nevertheless, survival time depends on the level of chlorination and the organic content of the water (Ahrens et al., 2022; Leung et al., 2007).
Effluents constitute an important factor in viral dissemination among poultry. Animal slurry (a liquid mixture of feces and urine added to litter, feed residues, washing water, and rainwater) contributes to AIV dissemination on poultry farms (Schmitz et al., 2020). Environmental samples collected from sewage may have high nucleic acid positivity rates of influenza (Bo et al., 2021; Guo et al., 2021). However, complex environments with high content of biological material (manure or feces) may retain infectivity for shorter periods than natural water (Schmitz et al., 2020). This is consistent with our findings where a low persistence risk was evidenced in wastewater.
The application of experimental results to field realities is complicated by the complexity and scale of these ecosystems (Stallknecht et al., 2010). Physicochemical properties such as temperature, pH, conductivity, ammonia concentration, and salinity can affect virus survival in different liquid environments (Keeler et al., 2014; Ramey et al., 2022; Schmitz et al., 2020). Nevertheless, other identified and unidentified factors prevailing in natural surface water may contribute to the effect of environmental persistence on AIV transmission dynamics among hosts (Martin et al., 2018; Nazir et al., 2010). The AIV subtype and its pathogenicity, bird density, UV light, and presence of biological compounds (freshwater crabs and microbial flora) were not evaluated in the MCDA. Likewise, further studies are required to assess the influence of viral, host, and biotic factors on AIV persistence and transmission in the wildlife-livestock interface.
Subtypes H1N1, H3N2, and H5N2 have been previously detected in wild birds in the study area (Gaytan-Cruz et al., 2020; Mateus-Anzola et al., 2020). However, no AIV has been detected in environmental samples at this wildlife/domestic bird interface. Negative samples may not reflect the true risk for AIV outbreaks (Belkhiria et al., 2018). Outbreaks of AIV in most tropical countries, such as Mexico, are mostly not detected due to limited surveillance infrastructure as well as the lack of standardization in sampling and reporting methods in both environmental and wild bird surveillance (Hood et al., 2020; Machalaba et al., 2015; Mateus-Anzola et al., 2021). Likewise, farmers from small-scale poultry farms usually do not report sick birds or unusual dead poultry to public health or agricultural authorities (Hall & Le, 2018; Hinjoy et al., 2023). This lack of detection and underreporting exacerbates the risk of unchecked AIV outbreaks in environments that enable the viral exchange between migratory waterfowl and domestic poultry.
In conclusion, the Atarasquillo wetland has eco-epidemiological factors that may enhance AIV survival and waterborne dissemination mainly in small-scale poultry farms close to the wetland. This MCDA provides valuable baseline information to identify the optimal environmental characteristics and high-risk epidemiological areas for AIV spreading as well as to develop early intervention strategies.
References
Adhikari, D., Chettri, A., & Barik, S. (2009). Modelling the ecology and distribution of highly pathogenic avian influenza (H5N1) in the Indian subcontinent. Current Science, 97(1), 73–78.
Ahrens, A. K., Selinka, H.-C., Mettenleiter, T. C., Beer, M., & Harder, T. C. (2022). Exploring surface water as a transmission medium of avian influenza viruses—Systematic infection studies in mallards. Emerging Microbes & Infections, 11(1), 1250–1261. https://doi.org/10.1080/22221751.2022.2065937
Barragán, M. P., & Figueroa, E. (2014). Riesgos a la salud humana relacionados con la expansión urbana en zonas susceptibles a inundación: Caso de estudio Guadalupe la Ciénega, Lerma [Tesis de Licenciatura en Ciencias Ambientales]. Universidad Autónoma del Estado de México.
Belkhiria, J., Hijmans, R. J., Boyce, W., Crossley, B. M., & Martínez-López, B. (2018). Identification of high risk areas for avian influenza outbreaks in California using disease distribution models. PLoS ONE, 13(1), e0190824. https://doi.org/10.1371/journal.pone.0190824
Bianchini, E. A., Bogiatto, R. J., Donatello, R. A., Casazza, M. L., Ackerman, J. T., De La Cruz, S. E. W., & Cline, T. D. (2022). Host correlates of avian influenza virus infection in wild waterfowl of the Sacramento Valley, California. Avian Diseases, 66(1), 20–28. https://doi.org/10.1637/aviandiseases-D-21-00071
Bo, H., Zhang, Y., Dong, L.-B., Dong, J., Li, X.-Y., Zhao, X., Li, Z., Shu, Y.-L., & Wang, D.-Y. (2021). Distribution of avian influenza viruses according to environmental surveillance during 2014–2018, China. Infectious Diseases of Poverty, 10(1), 60. https://doi.org/10.1186/s40249-021-00850-3
Bouwstra, R., Gonzales, J. L., de Wit, S., Stahl, J., Fouchier, R. A. M., & Elbers, A. R. W. (2017). Risk for low pathogenicity avian influenza virus on poultry farms, the Netherlands, 2007–2013. Emerging Infectious Diseases, 23(9), 1510–1516. https://doi.org/10.3201/eid2309.170276
Cao, C., Xu, M., Chang, C., Xue, Y., Zhong, S., Fang, L., Cao, W., Zhang, H., Gao, M., He, Q., Zhao, J., Chen, W., Zheng, S., & Li, X. (2010). Risk analysis for the highly pathogenic avian influenza in Mainland China using meta-modeling. Chinese Science Bulletin, 55(36), 4168–4178. https://doi.org/10.1007/s11434-010-4225-x
Chen, T., Tan, Y., Song, Y., Wei, G., Li, Z., Wang, X., Yang, J., Millman, A. J., Chen, M., Liu, D., Huang, T., Jiao, M., He, W., Zhao, X., Greene, C. M., Kile, J. C., Zhou, S., Zhang, R., Zeng, X., … Wang, D. (2023). Enhanced environmental surveillance for avian influenza A/H5, H7 and H9 viruses in Guangxi, China, 2017–2019. Biosafety and Health, S2590053622001811. https://doi.org/10.1016/j.bsheal.2022.12.006
Claes, G., Marché, S., Dewulf, J., Van Den Berg, T., & Lambrecht, B. (2014). An experimental model to analyse the risk of introduction of a duck-originated H5 low-pathogenic avian influenza virus in poultry through close contact and contaminative transmission. Epidemiology and Infection, 142(9), 1836–1847. https://doi.org/10.1017/S0950268813002793
CONANP. (2022). Áreas Naturales Protegidas Federales de México [Shapefile]. Secretaria del Medio Ambiente y Recursos Naturales. Comisión Nacional de Áreas Naturales Protegidas. http://www.conabio.gob.mx/informacion/metadata/gis/anp2022gw.xml?_httpcache=yes&_xsl=/db/metadata/xsl/fgdc_html.xsl&_indent=no
Dalziel, A. E., Delean, S., Heinrich, S., & Cassey, P. (2016). Persistence of low pathogenic influenza A virus in water: A systematic review and quantitative meta-analysis. PLoS ONE, 11(10), e0161929. https://doi.org/10.1371/journal.pone.0161929
Das Gupta, S., Barua, B., Fournié, G., Hoque, M. A., & Henning, J. (2022). Village and farm-level risk factors for avian influenza infection on backyard chicken farms in Bangladesh. Scientific Reports, 12(1), Article 1. https://doi.org/10.1038/s41598-022-16489-5
Delgado, M. G., & Sendra, J. B. (2004). Sensitivity analysis in multicriteria spatial decision-making: A review. Human and Ecological Risk Assessment: An International Journal, 10(6), 1173–1187. https://doi.org/10.1080/10807030490887221
Desvaux, S., Grosbois, V., Pham, T. T. H., Fenwick, S., Tollis, S., Pham, N. H., Tran, A., & Roger, F. (2011). Risk factors of highly pathogenic avian influenza H5N1 occurrence at the village and farm levels in the Red River Delta region in Vietnam: Case-control study on HPAI H5N1 in Northern Vietnam. Transboundary and Emerging Diseases, 58(6), 492–502. https://doi.org/10.1111/j.1865-1682.2011.01227.x
Domanska-Blicharz, K., Minta, Z., Smietanka, K., Marché, S., & van den Berg, T. (2010). H5N1 high pathogenicity avian influenza virus survival in different types of water. Avian Diseases, 54(s1), 734–737. https://doi.org/10.1637/8786-040109-ResNote.1
Dutta, P., Islam, A., Sayeed, Md. A., Rahman, Md. A., Abdullah, Md. S., Saha, O., Rahman, M. Z., Klaassen, M., Hoque, Md. A., & Hassan, M. M. (2022). Epidemiology and molecular characterization of avian influenza virus in backyard poultry of Chattogram, Bangladesh. Infection, Genetics and Evolution, 105, 105377. https://doi.org/10.1016/j.meegid.2022.105377
European Food Safety Authority, European Centre for Disease Prevention and Control, European Union Reference Laboratory for Avian Influenza, Adlhoch, C., Fusaro, A., Gonzales, J. L., Kuiken, T., Marangon, S., Mirinaviciute, G., Niqueux, É., Stahl, K., Staubach, C., Terregino, C., Broglia, A., & Baldinelli, F. (2023). Avian influenza overview December 2022–March 2023. EFSA Journal, 21(3). https://doi.org/10.2903/j.efsa.2023.7917
Ezell, B., Lynch, C., & Hester, P. (2021). Methods for weighting decisions to assist modelers and decision analysts: A review of ratio assignment and approximate techniques. Applied Sciences, 11(21), 10397. https://doi.org/10.3390/app112110397
Fang, L.-Q., de Vlas, S. J., Liang, S., Looman, C. W. N., Gong, P., Xu, B., Yan, L., Yang, H., Richardus, J. H., & Cao, W.-C. (2008). Environmental factors contributing to the spread of H5N1 avian influenza in Mainland China. PLoS ONE, 3(5), e2268. https://doi.org/10.1371/journal.pone.0002268
Ferrer, E., Alfonso, P., Ippoliti, C., Abeledo, M., Calistri, P., Blanco, P., Conte, A., Sánchez, B., Fonseca, O., Percedo, M., Pérez, A., Fernández, O., & Giovannini, A. (2014). Development of an active risk-based surveillance strategy for avian influenza in Cuba. Preventive Veterinary Medicine, 116(1–2), 161–167. https://doi.org/10.1016/j.prevetmed.2014.05.012
Garamszegi, L. Z., & Møller, A. P. (2007). Prevalence of avian influenza and host ecology. Proceedings of the Royal Society B: Biological Sciences, 274(1621), 2003–2012. https://doi.org/10.1098/rspb.2007.0124
Gaytan-Cruz, L., Mateus-Anzola, J., Montoya-Carrillo, C., Zarza, H., Garcia-Espinosa, G., & Ojeda-Flores, R. (2020). Phylogenetic characterization of a reassortant H5N2 influenza A virus from a resident Mexican duck (Anas diazi). Infection, Genetics and Evolution, 84, 104475. https://doi.org/10.1016/j.meegid.2020.104475
Ge, E., Haining, R., Li, C. P., Yu, Z., Waye, M. Y., Chu, K. H., & Leung, Y. (2012). Using knowledge fusion to analyze avian influenza H5N1 in East and Southeast Asia. PLoS ONE, 7(5), e29617. https://doi.org/10.1371/journal.pone.0029617
Guerrini, L., Paul, M. C., Leger, L., Andriamanivo, H. R., Maminiaina, O. F., Jourdan, M., Molia, S., Rakotondravao, R., & Chevalier, V. (2014). Landscape attributes driving avian influenza virus circulation in the Lake Alaotra region of Madagascar. Geospatial Health, 8(2), 445. https://doi.org/10.4081/gh.2014.33
Guo, J., Song, W., Ni, X., Liu, W., Wu, J., Xia, W., Zhou, X., Wang, W., He, F., Wang, X., Fan, G., Zhou, K., Chen, H., & Chen, S. (2021). Pathogen change of avian influenza virus in the live poultry market before and after vaccination of poultry in southern China. Virology Journal, 18(1), 213. https://doi.org/10.1186/s12985-021-01683-0
Hall, D. C., & Le, Q. B. (2018). Factors influencing mitigation of risk of waterborne disease in Vietnam among small scale integrated livestock farmers. Frontiers in Veterinary Science, 5, 154. https://doi.org/10.3389/fvets.2018.00154
Hall, J. S., Dusek, R. J., Nashold, S. W., TeSlaa, J. L., Allen, R. B., & Grear, D. A. (2020). Avian influenza virus prevalence in marine birds is dependent on ocean temperatures. Ecological Applications, 30(2). https://doi.org/10.1002/eap.2040
Hassan, M. M., Islam, A., Hasan, R. B., Rahman, Md. K., Webby, R. J., Hoque, Md. A., & El Zowalaty, M. E. (2020). Prevalence and distribution of avian influenza viruses in domestic ducks at the waterfowl-chicken interface in wetlands. Pathogens, 9(11), 953. https://doi.org/10.3390/pathogens9110953
Himsworth, C. G., Duan, J., Prystajecky, N., Coombe, M., Baticados, W., Jassem, A. N., Tang, P., Sanders, E., & Hsiao, W. (2020). Targeted resequencing of wetland sediment as a tool for avian influenza virus surveillance. Journal of Wildlife Diseases, 56(2), 397–408. https://doi.org/10.7589/2019-05-135
Hinjoy, S., Thumrin, P., Sridet, J., Chaiyaso, C., Smithsuwan, P., Rodchangphuen, J., Thukngamdee, Y., & Suddee, W. (2023). Risk perceptions of avian influenza among poultry farmers on smallholder farms along border areas of Thailand. Frontiers in Veterinary Science, 10, 1075308. https://doi.org/10.3389/fvets.2023.1075308
Hood, G., Roche, X., Brioudes, A., von Dobschuetz, S., Fasina, F. O., Kalpravidh, W., Makonnen, Y., Lubroth, J., & Sims, L. (2020). A literature review of the use of environmental sampling in the surveillance of avian influenza viruses. Transboundary and Emerging Diseases, 00, 1–17. https://doi.org/10.1111/tbed.13633
Huang, S., Tian, H., Wu, X., Zhou, S., Li, X., Zhang, T., Zhao, X., Wang, Y., Pei, Y., & Xu, B. (2016). Risk analysis of H5N1 highly pathogenic avian influenza in poultry at the Poyang Lake area, China. Environmental Earth Sciences, 75(11), 955. https://doi.org/10.1007/s12665-015-5111-2
INEGI. (2015). Guía para la interpretación de cartografía. Edafología. Escala 1:250 000. Serie III. https://www.inegi.org.mx/contenidos/productos/prod_serv/contenidos/espanol/bvinegi/productos/nueva_estruc/702825076221.pdf
INEGI. (2022). Áreas geoestadísticas municipal, 2021 [Shapefile]. Instituto Nacional de Estadística y Geografía. http://geoportal.conabio.gob.mx/metadatos/doc/html/mun21gw.html
INIFAP-CONABIO. (2008). Edafología [Map].
Islam, A., Islam, S., Amin, E., Shano, S., Samad, M. A., Shirin, T., Hassan, M. M., & Flora, M. S. (2022). Assessment of poultry rearing practices and risk factors of H5N1 and H9N2 virus circulating among backyard chickens and ducks in rural communities. PLoS ONE, 17(10), e0275852. https://doi.org/10.1371/journal.pone.0275852
Karasin, A. I., Brown, I. H., Carman, S., & Olsen, C. W. (2000). Isolation and characterization of H4N6 avian influenza viruses from pigs with pneumonia in Canada. Journal of Virology, 74(19), 9322–9327. https://doi.org/10.1128/JVI.74.19.9322-9327.2000
Keeler, S. P., Berghaus, R. D., & Stallknecht, D. E. (2012). Persistence of low pathogenic avian influenza viruses in filtered surface water from waterfowl habitats in Georgia, USA. Journal of Wildlife Diseases, 48(4), 999–1009. https://doi.org/10.7589/2011-11-314
Keeler, S. P., Dalton, M. S., Cressler, A. M., Berghaus, R. D., & Stallknecht, D. E. (2014). Abiotic factors affecting the persistence of avian influenza virus in surface waters of waterfowl habitats. Applied and Environmental Microbiology, 80(9), 2910–2917. https://doi.org/10.1128/AEM.03790-13
Kjær, L. J., Hjulsager, C. K., Larsen, L. E., Boklund, A. E., Halasa, T., Ward, M. P., & Kirkeby, C. T. (2022). Landscape effects and spatial patterns of avian influenza virus in Danish wild birds, 2006–2020. Transboundary and Emerging Diseases, 69(2), 706–719. https://doi.org/10.1111/tbed.14040
La Sala, L. F., Burgos, J. M., Blanco, D. E., Stevens, K. B., Fernández, A. R., Capobianco, G., Tohmé, F., & Pérez, A. M. (2019). Spatial modelling for low pathogenicity avian influenza virus at the interface of wild birds and backyard poultry. Transboundary and Emerging Diseases, tbed.13136. https://doi.org/10.1111/tbed.13136
Lang, A. S., Kelly, A., & Runstadler, J. A. (2008). Prevalence and diversity of avian influenza viruses in environmental reservoirs. Journal of General Virology, 89(2), 509–519. https://doi.org/10.1099/vir.0.83369-0
Leung, Y. H. C., Zhang, L.-J., Chow, C.-K., Tsang, C.-L., Ng, C.-F., Wong, C.-K., Guan, Y., & Peiris, J. S. M. (2007). Poultry drinking water used for avian influenza surveillance. Emerging Infectious Diseases, 13(9), 1380–1382. https://doi.org/10.3201/eid1309.070517
Li, X. H., Tian, H. D., Heiner, M., & Li, D. M. (2011). Global occurrence and spread of highly pathogenic avian Influenza virus of the subtype H5N1. Avian Diseases, 55(1), 21–28. https://doi.org/10.1637/9306-031710-Reg.1
Li, X.-L., Liu, K., Yao, H.-W., Sun, Y., Chen, W.-J., Sun, R.-X., de Vlas, S., Fang, L.-Q., & Cao, W.-C. (2015). Highly pathogenic avian influenza H5N1 in Mainland China. International Journal of Environmental Research and Public Health, 12(5), 5026–5045. https://doi.org/10.3390/ijerph120505026
Liu, Y. L., Wei, C. J., Yan, L., Chi, T. H., Wu, X. B., & Xiao, C. S. (2006). Analysis of spatial distribution and transmission characters for highly pathogenic avian influenza in Chinese mainland in 2004 (Q. Tong, W. Gao, & H. Guo, Eds.; p. 620011). https://doi.org/10.1117/12.682183
Machalaba, C. C., Elwood, S. E., Forcella, S., Smith, K. M., Hamilton, K., Jebara, K. B., Swayne, D. E., Webby, R. J., Mumford, E., Mazet, J. A. K., Gaidet, N., Daszak, P., & Karesh, W. B. (2015). Global avian influenza surveillance in wild birds: A strategy to capture viral diversity. Emerging Infectious Diseases, 21(4), e141415. https://doi.org/10.3201/eid2104.141415
Martin, G., Becker, D. J., & Plowright, R. K. (2018). Environmental persistence of influenza H5N1 is driven by temperature and salinity: Insights from a Bayesian meta-analysis. Frontiers in Ecology and Evolution, 6, 131. https://doi.org/10.3389/fevo.2018.00131
Martin, V., Pfeiffer, D. U., Zhou, X., Xiao, X., Prosser, D. J., Guo, F., & Gilbert, M. (2011). Spatial distribution and risk factors of highly pathogenic avian influenza (HPAI) H5N1 in China. PLoS Pathogens, 7(3), e1001308. https://doi.org/10.1371/journal.ppat.1001308
Mateus‐Anzola, J., Gaytan‐Cruz, L., Montoya‐Carrillo, C., Ivan Sánchez‐Betancourt, J., Zarza, H., Segura‐Velázquez, R., & Ojeda‐Flores, R. (2020). Molecular identification and phylogenetic characterization of influenza A virus at a wildlife–livestock interface in Mexico. Transboundary and Emerging Diseases, tbed.13962. https://doi.org/10.1111/tbed.13962
Mateus‐Anzola, J., Martínez‐López, B., Espinosa‐García, A. C., & Ojeda‐Flores, R. (2021). Global subtype diversity, spatial distribution patterns, and phylogenetic analysis of avian influenza virus in water. Transboundary and Emerging Diseases, tbed.14307. https://doi.org/10.1111/tbed.14307
McDuie, F., Casazza, M. L., Keiter, D., Overton, C. T., Herzog, M. P., Feldheim, C. L., & Ackerman, J. T. (2019). Moving at the speed of flight: Dabbling duck-movement rates and the relationship with electronic tracking interval. Wildlife Research, 46(6), 533. https://doi.org/10.1071/WR19028
McDuie, F., Matchett, E. L., Prosser, D. J., Takekawa, J. Y., Pitesky, M. E., Lorenz, A. A., McCuen, M. M., Overton Cory, T., Ackerman, J. T., De La Cruz, S. E. W., & Casazza, M. L. (2022). Pathways for avian influenza virus spread: GPS reveals wild waterfowl in commercial livestock facilities and connectivity with the natural wetland landscape. Transboundary and Emerging Diseases, 69(5), 2898–2912. https://doi.org/10.1111/tbed.14445
Müller-Theissen, M. L., Azziz-Baumgartner, E., Ortiz, L., Szablewski, C. M., Alvarez, D., Gonzalez-Reiche, A. S., Jara, J., Davis, C. T., & Cordon-Rosales, C. (2022). Influenza A virus circulation in backyard animals in the Pacific coast of Guatemala, 2013–2014. Zoonoses and Public Health, 69(7), 826–834. https://doi.org/10.1111/zph.12972
Nazir, J., Haumacher, R., Ike, A., Stumpf, P., Böhm, R., & Marschang, R. E. (2010). Long-term study on tenacity of avian influenza viruses in water (distilled water, normal saline, and surface water) at different temperatures. Avian Diseases, 54(s1), 720–724. https://doi.org/10.1637/8754-033109-ResNote.1
Nielsen, A. A., Jensen, T. H., Stockmarr, A., & Jørgensen, P. H. (2013). Persistence of low-pathogenic H5N7 and H7N1 avian influenza subtypes in filtered natural waters. Veterinary Microbiology, 166(3–4), 419–428. https://doi.org/10.1016/j.vetmic.2013.06.024
Odu, G. O. (2019). Weighting methods for multi-criteria decision making technique. Journal of Applied Sciences and Environmental Management, 23(8), 1449. https://doi.org/10.4314/jasem.v23i8.7
Paul, M. C., Gilbert, M., Desvaux, S., Rasamoelina Andriamanivo, H., Peyre, M., Khong, N. V., Thanapongtharm, W., & Chevalier, V. (2014). Agro-environmental determinants of avian influenza circulation: A multisite study in Thailand, Vietnam and Madagascar. PLoS ONE, 9(7), e101958. https://doi.org/10.1371/journal.pone.0101958
Perlas, A., Bertran, K., Abad, F. X., Borrego, C. M., Nofrarías, M., Valle, R., Pailler-García, L., Ramis, A., Cortey, M., Acuña, V., & Majó, N. (2023). Persistence of low pathogenic avian influenza virus in artificial streams mimicking natural conditions of waterfowl habitats in the Mediterranean climate. Science of the Total Environment, 863, 160902. https://doi.org/10.1016/j.scitotenv.2022.160902
Pfeiffer, D. U., Minh, P. Q., Martin, V., Epprecht, M., & Otte, M. J. (2007). An analysis of the spatial and temporal patterns of highly pathogenic avian influenza occurrence in Vietnam using national surveillance data. The Veterinary Journal, 174(2), 302–309. https://doi.org/10.1016/j.tvjl.2007.05.010
Pianosi, F., Beven, K., Freer, J., Hall, J. W., Rougier, J., Stephenson, D. B., & Wagener, T. (2016). Sensitivity analysis of environmental models: A systematic review with practical workflow. Environmental Modelling & Software, 79, 214–232. https://doi.org/10.1016/j.envsoft.2016.02.008
Ramey, A. M., Reeves, A. B., Lagassé, B. J., Patil, V., Hubbard, L. E., Kolpin, D. W., McCleskey, R. B., Repert, D. A., Stallknecht, D. E., & Poulson, R. L. (2022). Evidence for interannual persistence of infectious influenza A viruses in Alaska wetlands. Science of the Total Environment, 803, 150078. https://doi.org/10.1016/j.scitotenv.2021.150078
Schmitz, A., Pertusa, M., Le Bouquin, S., Rousset, N., Ogor, K., LeBras, M.-O., Martenot, C., Daniel, P., Hontecillas, A. B. C., Scoizec, A., Morin, H., Massin, P., Grasland, B., Niqueux, E., & Eterradossi, N. (2020). Natural and experimental persistence of highly pathogenic H5 influenza viruses in slurry of domestic ducks, with or without lime treatment. Applied and Environmental Microbiology, 86(24), e02288-20. https://doi.org/10.1128/AEM.02288-20
SEMARNAT-CONANP. (2018, October). Programa de manejo. Área de protección de flora y fauna Ciénegas de Lerma. https://simec.conanp.gob.mx/pdf_libro_pm/20_libro_pm.pdf
Shafiq, A., Arshad, A., Qurashi, Z.-U.-A., Khalid, Z., Ul Huda, N., Noman, M., Ilyas, M., Bin Rashid, H., Mehmood, A. K., Hasan, S., Ain, Q., Amir, S., Shahid, S., Sadiq, S., & Chaudhry, M. (2021). Estimation of percentage of morbidity and mortality due to avian influenza (H9) at commercial poultry layer farms of Lahore. Thai Journal of Veterinary Medicine, 51(4), 753–757. https://doi.org/10.14456/tjvm.2021.91
Shimizu, Y., Hayama, Y., Yamamoto, T., Murai, K., & Tsutsui, T. (2018). Matched case-control study of the influence of inland waters surrounding poultry farms on avian influenza outbreaks in Japan. Scientific Reports, 8(1), 3306. https://doi.org/10.1038/s41598-018-21695-1
Shoham, D., Jahangir, A., Ruenphet, S., & Takehara, K. (2012). Persistence of avian influenza viruses in various artificially frozen environmental water types. Influenza Research and Treatment, 2012, 1–11. https://doi.org/10.1155/2012/912326
Si, Y., de Boer, W. F., & Gong, P. (2013). Different environmental drivers of highly pathogenic avian Influenza H5N1 outbreaks in poultry and wild birds. PLoS ONE, 8(1), e53362. https://doi.org/10.1371/journal.pone.0053362
Stallknecht, D. E., Goekjian, V. H., Wilcox, B. R., Poulson, R. L., & Brown, J. D. (2010). Avian influenza virus in aquatic habitats: What do we need to learn? Avian Diseases, 54(s1), 461–465. https://doi.org/10.1637/8760-033109-Reg.1
Stenkamp-Strahm, C., Patyk, K., McCool-Eye, M. J., Fox, A., Humphreys, J., James, A., South, D., & Magzamen, S. (2020). Using geospatial methods to measure the risk of environmental persistence of avian influenza virus in South Carolina. Spatial and Spatio-Temporal Epidemiology, 34, 100342. https://doi.org/10.1016/j.sste.2020.100342
Thanapongtharm, W., Van Boeckel, T. P., Biradar, C., Xiao, X., & Gilbert, M. (2013). Rivers and flooded areas identified by medium-resolution remote sensing improve risk prediction of the highly pathogenic avian influenza H5N1 in Thailand. Geospatial Health, 8(1), 193. https://doi.org/10.4081/gh.2013.66
Tran, A., Goutard, F., Chamaillé, L., Baghdadi, N., & Lo Seen, D. (2010). Remote sensing and avian influenza: A review of image processing methods for extracting key variables affecting avian influenza virus survival in water from earth observation satellites. International Journal of Applied Earth Observation and Geoinformation, 12(1), 1–8. https://doi.org/10.1016/j.jag.2009.09.014
Van Boeckel, T. P., Thanapongtharm, W., Robinson, T., Biradar, C. M., Xiao, X., & Gilbert, M. (2012). Improving risk models for avian influenza: The role of intensive poultry farming and flooded land during the 2004 Thailand Epidemic. PLoS ONE, 7(11), e49528. https://doi.org/10.1371/journal.pone.0049528
Walsh, M. G., Amstislavski, P., Greene, A., & Haseeb, M. A. (2017). The Landscape epidemiology of seasonal clustering of highly pathogenic avian influenza (H5N1) in domestic poultry in Africa, Europe and Asia. Transboundary and Emerging Diseases, 64(5), 1465–1478. https://doi.org/10.1111/tbed.12537
Wang, X., Wang, Q., Cheng, W., Yu, Z., Ling, F., Mao, H., & Chen, E. (2017). Risk factors for avian influenza virus contamination of live poultry markets in Zhejiang, China during the 2015–2016 human influenza season. Scientific Reports, 7(1), Article 1. https://doi.org/10.1038/srep42722
Wang, Y., Li, P., Wu, Y., Sun, X., Yu, K., Yu, C., & Qin, A. (2014). The risk factors for avian influenza on poultry farms: A meta-analysis. Preventive Veterinary Medicine, 117(1), 1–6. https://doi.org/10.1016/j.prevetmed.2014.06.008
Zepeda, C., Lot, A., Nemiga, X. A., & Manjarrez, J. (2014). Seed bank and established vegetation in the last remnants of the Mexican Central Plateau wetlands: The Lerma marshes. Revista De Biología Tropical, 62, 455–472.
Zepeda-Gómez, C., Lot-Helgueras, A., Nemiga, X. A., & Madrigal-Uribe, D. (2012). Floristics and diversity of the Lerma River wetlands in the State of Mexico. Acta BotáNica Mexicana, 89, 23–49.
Zhang, H., Li, Y., Chen, J., Chen, Q., & Chen, Z. (2014). Perpetuation of H5N1 and H9N2 avian influenza viruses in natural water bodies. Journal of General Virology, 95(7), 1430–1435. https://doi.org/10.1099/vir.0.063438-0
Zhao, J., Smith, T., Lavigne, M., Aenishaenslin, C., Cox, R., Fazil, A., Johnson, A., Sanchez, J., & Hermant, B. (2022). A Rapid literature review of multi-criteria decision support methods in the context of one health for all-hazards threat prioritization. Frontiers in Public Health, 10, 861594. https://doi.org/10.3389/fpubh.2022.861594
Acknowledgements
This research belongs to the PAPIIT-DGAPA project no IN222119. Jessica Mateus-Anzola and Liliana Gaytan-Cruz are doctoral students of the “Posgrado en Ciencias de la Producción y de la Salud Animal”, Universidad Nacional Autónoma de México (UNAM). The authors are grateful to the “Consejo Nacional de Humanidades, Ciencias y Tecnologías (CONAHCYT)” for postgraduate fellowship (N° 785318 and 737947). We also thank “Laboratorio Nacional de Ciencias de la Sostenibilidad” and Marco Antonio Tapia for supporting this project with their expertise. We greatly appreciate the participation and cooperation of farmers and boat operators.
Funding
This work was funded by the PAPIIT-DGAPA project (number IN222119) and the NSF award (number 2137235).
Author information
Authors and Affiliations
Contributions
This work was carried out in collaboration between all authors. JM: writing—original draft, software, methodology, formal analysis, project administration, LG: methodology, project administration, writing—review and editing. BM: formal analysis, writing—review and editing. ACE: methodology, writing—review and editing. RO: project administration, funding acquisition, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mateus-Anzola, J., Gaytan-Cruz, L., Espinosa-García, A.C. et al. Risk for Waterborne Transmission and Environmental Persistence of Avian Influenza Virus in a Wildlife/Domestic Interface in Mexico. Food Environ Virol (2024). https://doi.org/10.1007/s12560-024-09608-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12560-024-09608-0