Abstract
Introduction
Hadron therapy is today an established modality in cancer radiation therapy that, world-wide, is available in about hundred centres. About 90% of them are devoted to proton therapy and the rest also to carbon ion therapy. Based on the superior ballistic and radiobiological properties of fast ions, this discipline experienced a remarkable growth in the last 30 years.
Purpose
This paper reviews the history of hadron therapy starting from the initial work done at Berkeley in the 30’s.
Methods
Fast neutron therapy is not forgotten but the main accent is on the parallel development of proton and carbon ion therapy. For space reasons pion therapy, to which a lot of efforts have been devoted without success, is not discussed.
Results
The activities of the main protagonists in USA, Japan and Europe are shortly presented so that the reader can appreciate the relevance of the contribution given by each of them to the advancement of the field.
Conclusions
The review ends in 2005, when the carbon ion centres HIT in Heidelberg and CNAO in Pavia were under construction, with the aim of putting at the disposal of European citizens advanced treatments of radioresistant tumours, which cannot be effectively treated neither with X rays nor with protons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introductory remarks
‘Hadron therapy’ or ‘hadrontherapy’ (‘hadronthérapie’ in French, ‘hadronentherapie’ in German, ‘adroterapia’ in Italian and ‘hadronterapia’ in Spanish) are collective words which cover all forms of radiation therapy which use beams of particles made of quarks: neutrons, protons, antiprotons, helium ions (i.e. alphas), lithium ions, boron ions, carbon ions, oxygen ions etc. I greatly prefer “hadron therapy” to “particle therapy”, which is also widely used, because in physics.
-
the photons of X-rays, used in all Radiation departments, are also ‘particles’, more precisely ‘force-particles’,
-
the electrons, used in many Radiotherapy departments are also ‘particles’, more precisely ‘matter-particles’,
and neither photons nor electrons are the bullets of this kind of radiation therapy.
Moreover, when speaking of therapy with ion beams, I insist that one should use the terms ‘light ions’ and not ‘heavy ions’ because, according to the International Commission on Radiation Units – ICRU – “all ions with a charge number Z smaller than 10 will be referred to as ‘light ions’ ”.
A final remark: ‘heavy particle therapy’, unfortunately rarely used, is scientifically correct.
2 Lawrence cyclotrons and neutron therapy
Hadron therapy has its roots in the 1930 invention of the cyclotron by Ernest Lawrence. In 1935 he asked his brother John, who was a medical doctor at Yale, to join him in Berkeley and use the new powerful accelerator for medical purposes [1]. This is the reason for which I have chosen the year 1935 as the beginning of this short history of hadron therapy (Fig. 1).
The two applications were the production of radioisotopes and, later, the therapeutical use of fast neutron beams. Many years later Ed McMillan remarked that “Lawrence thought you could do anything with a cyclotron” [2].
Soon after the arrival of John in Berkeley, Ernest Lawrence and his brother started studying the effects of fast neutrons on biological systems. Following a paper by Gordon Locher [3], who in 1936 underlined the therapeutic potentialities of both fast and slow neutrons, at the end of September 1938 the first patients were treated with neutrons produced by the 37-inch cyclotron through the reaction of 8 MeV deuterons on a beryllium target (Fig. 2).
This initial investigation, which involved 24 patients using single fractions, was deemed successful and paved the way for the building of a special 60-inch Crocker Medical Cyclotron, financed by a very rich Regent of Berkeley University. With it, Robert Stone and his associates used fractionated dosages of neutrons generated by sixteen 16 MeV deuterons on beryllium to treat patients until 1943, when the cyclotron was taken over for use in the atomic bomb project. The technique was primitive, and the doses given to healthy tissues were too high, so that in 1948 Stone evaluated the effects on 226 patients and concluded: “Neutron therapy as administered by us has resulted in such bad late sequels in proportion to the few good results that it should not be continued” [4].
In 1965 Mary Catterall at the Hammersmith Hospital conducted Phase 3 trials with better neutron beams and more fractions and obtained good results for superficial adenocarcinomas [5]. Following this revival many neutron facilities were built and patients have been treated since then so that, in a recent review paper, one can read “Although fast neutrons have a bad name due to their controversial clinical track record, their overall potential in cancer treatment seems to be underestimated. They are interesting for radioresistant superficial tumours. Fast neutrons are guaranteed a limited, but special, place in modern radiation oncology” [6].
3 The beginnings of therapy with charged hadrons
In 1945, Robert Rathbun “Bob” Wilson — who was a student of Lawrence and much later became the Fermilab founder and director — was hired as an associate professor at Harvard and designed a new 160 MeV cyclotron which, after many years of exploitation for nuclear physics experiments, was used in 1961 to irradiate patients. But already in 1946 Wilson (Fig. 3) had proposed the use of proton beams in radiation oncology [7]. In fact, he had measured at the Berkeley Cyclotron depth profiles with a significant increase in dose at the end of particle range, the so-called Bragg peak that Wilson proposed to “spread” with modulator wheels. It is interesting to remark that in his seminal paper Wilson discussed mainly protons but mentioned also alpha particles and carbon ions.
Two years later, researchers at the Lawrence Berkeley Laboratory (LBL) conducted extensive studies on protons and confirmed the predictions made by Wilson. In 1954, the first patient was treated at Berkeley with protons, followed by helium treatment in 1957 and neon ions in 1975. In these initial treatments the beam was distributed over the target volume using “passive” shaping systems (like scatterers, compensators and collimators that were adapted from the conventional photon therapy) and thus treating these particles as photons without making use of their most important characteristics, the electric charge, which makes their beams easy to guide by means of magnetic fields.
The first treatments on humans consisted of irradiation to destroy the pituitary gland in patients with metastatic breast cancer that was hormone sensitive. The pituitary was a natural site for the first treatments because the glands’ location was easily identified by means of standard X-ray films [8]. Between 1954 and 1974 at Berkeley, under the leadership of Cornelius Tobias and John Lawrence, about 1000 pituitary glands and pituitary tumours were treated with protons. In 1957, the first tumour was irradiated with protons at the Uppsala cyclotron by Börje Larsson (Fig. 4), who got his Ph.D. in 1962 by discussing the thesis: “Application of a 185 MeV Proton Beam to Experimental Cancer Therapy and Neurosurgery: A Biophysical Study” [9].
4 The Harvard cyclotron
The facility that made the largest impact on the development of proton therapy is the Harvard cyclotron (Fig. 5a). Its story is long and very interesting [10].
(a) A famous picture of the just completed Harvard cyclotron. Many years later Norman Ramsey (right) was awarded the Nobel prize, together with Wolfang Paul. (b) Herman Suit (right) and Michael Goitein in 2003, when Goitein received the Medal of the American Society for Radiation Oncology.
Harvard built the first such machine in 1937, but the federal government drafted it during World War I: it was taken apart and shipped to Los Alamos in 1943, for service in designing the first atomic bombs. As mentioned above, while designing the machine that should substitute the removed one, Bob Wilson thought of using it for medical purposes. However, the staff of the Harvard Cyclotron Facility became interested in using protons for medical treatments only after proton therapy was started in the ’50 at both LBL and Uppsala.
The early work was limited due to the inability to perform 3-D imaging and reliance on facilities primarily dedicated to physics research. With the development of the CT scanner, improved target definition allowed for the treatment of almost any site in the body. Overall, three groups of radiation oncologists worked for many decades with Harvard physicists on three clinical studies: neurosurgery for intracranial lesions (3687 patients), eye tumours (2979 patients) and head–neck tumours (2449 patients). In 1961, Raymond Kjellberg, a young neurosurgeon at Massachusetts General Hospital in Boston, developed neurosurgery. The main people who did work on eye tumours and malformations were Ian Constable and Evangelos Gragoudas of the Massachusetts Eye and Ear Hospital. The successes obtained on large brain tumours are due to Herman Suit and Michael Goitein (Fig. 5b) and their colleagues of the Radiation Medicine Department of Massachusetts General Hospital [11].
The results obtained (particularly for eye melanoma and for chordomas and chondrosarcomas of the base of the skull) convinced many radiation oncologists of the superiority of protons with respect to X-rays for tumours that are close to organs at risk.
5 Proton therapy from Harvard to Loma Linda
As shown in Table 1, soon after the start-up of the Harvard facility other nuclear physics laboratories in USSR and Japan, and eventually in Switzerland, setup proton beams for therapy.
All the facilities listed in the table were located in physics laboratories and the irradiation conditions were far from ideal. Overall, these developments covered more about 40 years but, finally, about 14 500 patients had been treated.
In many places and many times, it was felt and said that the field would not develop without a dedicated facility. However, only at the end of the 80’s the first hospital-based centre was built for the Loma Linda University Center (California), because of the determination of James Slater who initiated a collaboration with Fermilab, founded and directed for many years by Bob Wilson. Loma Linda University obtained federal support for the $80 million proton therapy facility and, in 1986, Loma Linda University and Fermilab signed an agreement, with which Fermilab took on the task of building the $25 million proton accelerator. The first patient was treated in 1990 in the Loma Linda centre, featuring three gantries rotating around the patient bed. In the same year, Medicare approved the coverage of this new modality for treating cancer.
A smooth conversion from a physics laboratory to a hospital facility took place in Japan. The University of Tsukuba started proton clinical studies in 1983 using a synchrotron constructed for physics studies at the High Energy Accelerator Research Organization KEK (Table 1). In 2000, a new facility was completed adjacent to the University Hospital and equipped with a synchrotron and two rotating gantries built by Hitachi: clinical treatments started in September 2001.
6 The first international conference on hadron therapy
In 1993-94 the development of hadron therapy greatly accelerated. Luckily, I could actively participate to this exciting period because in 1991 – being a CERN staff member spokesperson of the international Collaboration DELPHI working for more than ten years at the Large Electron-Positron collider LEP – I decided to leave particle physics and devote myself to hadron therapy [12]. TERA foundation was created in September 1992 and in December I went, with Guido Petrucci and Marco Silari, to PSI (Villigen) to meet Börje Larsson and his collaborators. The year before Larsson had been offered a professorship in Medical Radiation Biology at Zurich University and, when I met him, was leader of the Institute for Medical Radiobiology of the university of Zurich and of PSI and of the proton therapy group. In the next months we had many exchanges, and we became friends.
As a new-comer I was surprised to learn that hadron therapy advances were discussed in regular PTCOG meetings but no large Conference with refereed proceedings had ever been organized. This Larsson and I did together in October 1993 in Como, where I had just joined the Second Science Faculty of Milano University as full professor in Medical Physics. Practically all the protagonists of hadron therapy from Europe, Japan, USA, and USSR were present and the 750 pages volume of the proceedings “Hadrontherapy in Oncology” is still a reference in this field [13].
This book contains - on top of historical reviews, as the one of Ref. 11, and papers describing the treatments delivered in all existing hadron therapy centres at that time - presentations of all the developments that have shaped hadron therapy in the following decades:
-
1)
the first hospital-based proton facility at Loma Linda [14];
-
2)
the treatment of uveal melanomas at PSI [15];
-
3)
the description of the GSI ‘pilot project’ for carbon ion therapy [16];
-
4)
the HIMAC project promoted by NIRS, which in 1994 treated its first patient with carbon ions [17];
-
5)
the advantages of beam scanning and the design of advanced proton gantries [18];
-
6)
the technique of spot scanning with protons at PSI [19];
-
7)
the Italian Hadrontherapy Programme based on the National Centre CNAO, the compact accelerator project PACO - with the cyc-linac proposal - and the network Rete Italiana Trattamenti Adroterapici RITA, connecting many centres [20];
-
8)
the addition of a medical facility to the spallation source project called AUSTRON [21];
-
9)
the design of the IBA proton cyclotron and gantry [22];
-
10)
the new proton facility to be built at Massachusetts General Hopital [23].
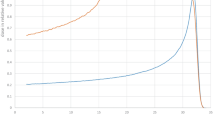
Shortly after the Como Conference, the MGH group choose the IBA system [22] as provider of the new proton facility. As indicated in Fig. 6a, this commercial system was the first of 13 centres built till 2005 and opened the way to IBA to become the market leader in proton therapy.
Fig. 7 shows the world status of proton therapy in 2005, at the end of the period reviewed in this paper.
7 The beginnings of light-ion therapy: USA and Japan
Heavier ions than protons, such as helium and later-on argon, first came into use at Berkeley in 1957 and 1975, respectively. At the 184-inch cyclotron patients received treatments to the pituitary gland with helium beams, the lateral spread and range straggling being much smaller than in the proton case. The rationale of these treatments was the reduction of breast tumours when the pituitary would be inactivated.
About 20 years later, heavier ion beams were used at the ‘Bevalac’ [24], which is schematically presented in Fig. 8.
The purpose was to increase the effectiveness in controlling hypoxic and otherwise radioresistant tumours, i.e. tumours that need deposited doses 2–3 times higher if they are to be controlled with either photons or protons. But problems arose owing to non-tolerable side effects in the normal tissues. After a few irradiations of some 20 patients with argon, Cornelius Tobias and collaborators used lighter ions, first silicon ions for two patients and then neon, for 433 patients until the Bevalac stopped operation in 1993. Only towards the end of the program it was found that the neon charge (Z = 10) is too large and undesirable effects are produced in the traversed and downstream healthy tissues [25].
The carbon choice was made in Japan by Hirohito Tsujii, Yasuo Hira and collaborators, who proposed and built HIMAC in the Chiba Prefecture [26]. As mentioned above, in 1994 the first patient was treated with a carbon ion beam of energies smaller than 400 MeV/u, corresponding to a maximum water range of 27 cm. Eight years later, Hirohiko Tsujii and collaborators wrote in Ref [27]: “By August 2006 a total of 2,867 patients had been entered into Phase I/II or Phase II studies and analysed for toxicity and local tumour response. Tumours that appear to respond favourably to carbon ions include locally advanced tumours and those with histologically non-squamous cell type of tumours such as adenocarcinoma, adenoid cystic carcinoma, malignant melanoma hepatoma, and bone/soft tissue sarcoma.”
In 2001 the Hyogo Ion Beam Medical Center (HIBMC), built by Mitsubishi Electric, was completed as the world’s first institution where both proton and carbon-ion radiotherapy can be performed [28]. In 2007 Mitsuyuki Abe, the world-known radiation oncologist who was responsible for the construction allocated to Mitsubishi Electric, wrote a beautiful paper on the 1400 patients treated between April 2001 and February 2007 [29].
8 Light-ion therapy in Europe
In 1987, in Europe, an important initiative was launched to create a full-fledged European light-ion therapy centre. The needed hadron beams were defined in a series of expert meetings. The European Light Ion Medical Accelerator (EULIMA) project, financed by the European Commission, was led by Pierre Mandrillon and involved many European laboratories and centres. Initially the project, by making use of the Berkeley experience, foresaw the use of O + 8 ions, but during the study a worldwide consensus was reached that a better choice is C + 6. The core of the project group was hosted by CERN. A paper describes the two 400 MeV/u accelerators, a superconducting cyclotron, and a synchrotron, which have been studied together with the active dose spreading system and a rotating gantry [30].
Eventually, the EULIMA project management board recommended the synchrotron option as the accelerator for EULIMA but, unfortunately, such a European therapy centre was never built because there were disagreement among the participating groups and the European funds ended.
In 1993, the German nuclear physicist and radiobiologist Gerhard Kraft (Fig. 9) and medical doctor Jurgen Debus obtained the approval for the already mentioned construction at GSI of a carbon ion “pilot project” [16]. A treatment area was equipped with a precision couch and a horizontal ion beam of energy smaller than 400 MeV/nucleon and produced by the GSI synchrocyclotron. Patient treatments started in 1997 and about 450 patients had been treated with carbon ion beams when in 2008 the facility was stopped [31].
Based on the successes of the pilot project, the Heidelberg Ion Therapy Centre (HIT) was approved in 2001 by the German authorities and the civil engineering work could start in November 2003. The first treatment took place in 2009. This centre features the first carbon ion gantry, which is 25 m long; with this gantry the first patient was irradiated in fall 2012 [31].
At the end of 1995, to focus the best available CERN competences on the design of a top-level facility, Mainard Regler (of the Austron project) and I proposed to the management to initiate, under the direction of Philip Bryant, the Proton Ion Medical Machine Study (PIMMS) centred on the design of a synchrotron and a system of beam lines optimized for the treatment of deep-seated tumours with collimated beams of carbon ions, protons and other light ions (Fig. 10). Since the beginning PIMMS was conceived as a tool-kit from which potential user could extract and use any subproject of interest.
From 1996 to 2000, the study was carried out at CERN with the part-time participation of many members of the PS (Proton Synchrotron) Division. The design study was closed at the end of 2000 with the publication of two reports [32, 33]. Figure 10 presents the PIMMS layout together with some technical information on the PIMMMS 400 MeV/nucleon synchrotron.
During the years 1998–2003 TERA used many parts of PIMMS and introduced modifications and improvements to the original design of the synchrotron and of the beam lines developed by PIMMS, producing the ‘PIMMS/TERA’ design. As shown in Fig. 11, the linac (for protons and carbon ions) is located inside the ring and the three treatment lines follow a fan-out magnet and thus are very short with respect to the original proposal, which results in a much smaller footprint than the one of PIMMS (Fig. 10).
In May 2001, the Italian Health Minister Umberto Veronesi created the CNAO Foundation; the founders are TERA, the two large University hospitals of Milano (Ospedale Maggiore) and Pavia (Ospedale San Matteo), two Milano oncological hospitals (INT and IEO) and the neurological Institute ‘Besta’. At the end of 2003 TERA passed to CNAO Foundation its core group (led by Sandro Rossi and formed of 15 staff members and 6 experts) together with 2000 pages of drawings and detailed technical specifications. In 2004 the Italian National Institute of Nuclear Physics INFN became institutional participant of CNAO and started to contribute to the construction of the centre in Pavia, the town chosen by the Government as CNAO seat [34, 35].
Between the Como meeting of fall 1993 and 2004 the European hadron therapy scene had completely changed so that in 2005 Gerhard Kraft and I wrote a much-quoted paper, published in Reports on Progress in Physics [36], which described the basis of carbon ion therapy and the German and Italian programs with their centres HIT in Heidelberg and CNAO in Pavia.
The construction of CNAO started in Pavia in April 2005 and the first patient was treated in September 2011. Figure 12 shows the status of the construction in January 2006.
I conclude this review by recalling that the first patients were treated at HIT in 2009 and at CNAO in 2011 and that, by the end of 2023, 8000 patients had been treated at HIT and 4500 patients at CNAO; about half of these 12,500 patients had been irradiated with carbon ions [37]. At the same date, the seven Japanese centres had treated with carbon ions about 38,000 patients, of which 15,000 at HIMAC. For comparison, worldwide 310,000 patients had been irradiated with proton beams [37].
Recently it has been announced by Mayo Clinic that the first American centre accelerating carbon ions, with a Hitachi synchrotron, will be opened in 2027 [38], 25 years after the end of the seminal studies performed at LBNL on the ion effectiveness in curing radioresistant tumours.
Data availability
There is no original data in this manuscript.
References
Childs H. An American genius: the life of Ernest Orlando Lawrence. E. P. Dutton; 1968.
Jungerman JA. EarlyNuclearPhysicsResearchandtheFormationofCrockerNuclearLaboratory, see https://cyclotron.crocker.ucdavis.edu/building-cyclotron/, last visited 06.01.24.
Locher GL. Biological effects and therapeutic possibilities of neutrons, am. J Roentgenol. 1936;36:1.
Stone RS. Neutron therapy and specific ionization, am. J Roentgenol. 1948;59:771.
Catterall M. First randomized clinical trial of fast neutrons compared with photons in advanced carcinoma of the head and neck. Clin Otolaryngol Allied Sci. 1977;2:359–72.
Gordon K, Gulidov I, Fatkhudinov T, Koryakin S, Kaprin A. Fast and furious. Fast neutron therapy in cancer treatment. Int j Part Ther. 2022;9(2):59–69.
Wilson RR. Radiological use of fast protons. Radiology. 1946;47:487.
Lawrence JH, Tobias CA, Born JL, Wang CC, Linfoot JH. Heavy-particle Irradiation Neoplast Neurologic Disease J Neurosur. 1962;19(9):717.
Larsson B. On the application of a 185 MeV proton beam to experimental cancer therapy and neurosurgery: A biophysical study, Acta Universitatis Upsaliensi, Abstracts of Uppsala Dissertations in Science, No 9 (1962).
Wilson R. A brief history of the Harvard University Cyclotron. Harvard Univ. Press; 2003.
Raju MR. Hadrontherapy in an historical and international perspective. Ref 12, 67–82.
I have explained the motivations of this decision. And described the basis of particle physics and of hadron therapy in: U. Amaldi, ‘Particle accelerators: from Big Bang physics to hadron therapy’. Springer; 2015. pp. 1–284. https://link.springer.com/book/. https://doi.org/10.1007/978-3-319-08870-9. The pdf of this book is freely available.
Amaldi U, Larsson Eds B. ‘Hadrontherapy in Oncology’, Elsevier, Excerpta Medica Int. Congress Series 1077, 1994, pp. 1-754.
Slater JM, Archambeau JO, Dicello JF, Slate JD. Proton Beam irradiation: toward routine clinical utilization. Ref 13, 130–7.
Egger E, Zofragos L. Proton Beam irradiation of uveal melanomas at PSI: latest results. Ref 13, 145–9.
Kraft G, Boehne D, Haberer T et al. The Darmstadt Program HITAG: heavy ion therapy at GSI. Ref 13, 217–28.
Kawachi K, Yamada S, Hirao Y et al. Heavy ion medical accelerator facility in Japan. Ref 13, 229–40.
Pedroni E. Advantages of beam scanning and requirements of hadrontherapy facilities. Ref 13, 434–52.
Markovits CH. Spot scanning with protons at PSI: experimental results and treatment planning, Ref. 13, 462–470.
Amaldi U. The Italian Hadrontherapy Project Ref 13, 45–58.
Bryant P, Poetter R, Regler M et al. Ion cancer Therapy Res as part AUSTRON Project Ref 13, 390–9.
Jongen Y, Beeckmann W, Laisné A et al. Cyclotron-based Protontherapy Syst Including new Des Large Throw Gantries Ref 13, 155–64.
Smith A, Goitein M, Flanz J et al. The Mass Gen Hosp Northeast Protontherapy Cent Ref 13, 138–44.
Ghiorso A, Grunder H, Hartsough W, et al. The Bevalac - an economical facility for very energetic heavy particle research. IEEE Trans Nucl Sci. 1973;20:155–8. https://ieeexplore.ieee.org/abstract/document/4327069/similar#similar.
Castro JR. Heavy ion therapy: The BEVALAC epoch, Ref 13, 208 – 144.
Hirao Y, Ogawa H, Yamada S, et al. Heavy ion synchrotron for medical use – himac project at NIRS – Japan. Nucl Phys A. 1992;538:541.
Tsujii H, Mizoe J, Kamada T, et al. Clinical results of carbon ion therapy at NIRS. J Rad Res. 2007;48(SupplA):A1–1. https://doi.org/10.1269/jrr.48.A1.
Ishi Y, Itano A, Yamamoto Y et al. October, Beam Commissioning of Synchrotron Ring at Hyogo Ion Beam Medical Center, Proc. 13th Symposium Acc Sci and Tech, Suita, Osaka, 2001: Beam Commissioning of Synchrotron Ring at Hyogo Ion Beam Medical Center: www.pasj.jp/web_publish/sast2001/9P02.pdf.
Abe M. Charged particle radiotherapy at the Hyoigo Ion Beam Medical Center: characteristics, technology and clinical resultts. Proc Jpn Acad Ser B Phys Biol Sci. 2007;83:151–63. www.ncbi.nlm.nih.gov/pmc/articles/PMC3855203/.
Mandrillon P, Carli C. G.Cesari Feasibility study of the EULIMA light ion medical accelerator, Proc. EPAC 92, pp. 179–181: https://accelconf.web.cern.ch/e92/PDF/EPAC1992_0179.PDF.
Jäkel O, Kraft G, Karger CP. The history of ion beam therapy in Germany. Z Med Phys. 2022;32:6–22.
Badano L, Benedikt M, Bryant PJ, Crescenti M, Holy P, Knaus P, Meier A, Pullia M, Rossi S. Proton-Ion Medical Machine Study (PIMMS) — part I, CERN/PS 99 – 010 DI (March 1999).
Badano L, Benedikt M, Bryant PJ, Crescenti M, Holy P, Knaus P, Meier A, Pullia M, Rossi S. Proton-Ion Medical Machine Study (PIMMS) — Part II, CERN/PS 00–007 DR (July 2000).
Rossi S. The status of CNAO, Eur. Phys J Plus. 2011;126:78.
Amaldi U. and G. Magrin Eds, Mercurio, 2011.
Amaldi U, Kraft G. Radiotherapy with beam of carbon ions. Rep Prog Phys. 2005;68:1861. https://doi.org/10.1088/0034-4885/68/8/R04. https://iopscience.iop.org/article/.
https://www.ptcog.site/images/Statistics/Patientstatistics-updateDec2022_Dec2023.pdf.
Kramer D. Slow but steady progress seen for carbon-ion cancer therapy. Phys Today. 2022;75:22–5. https://pubs.aip.org/physicstoday/article-abstract/75/9/22/2845460/Slow-but-steady-progress-seen-for-carbon-ion?redirectedFrom=fulltext.
Funding
The author(s) have received no funding for this article.
Open access funding provided by CERN (European Organization for Nuclear Research)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
No Ethical Approval was required for this article.
Consent to participate
No Consent to Participate was required for this article.
Consent for publish
No Consent to Publish was required for this article.
Competing interests
The author(s) have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amaldi, U. Evolution of hadron therapy from 1935 to 2005: a personal view. Health Technol. (2024). https://doi.org/10.1007/s12553-024-00894-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12553-024-00894-z