Abstract
Background
Baltic States remains one of the few regions in the Europe without a dedicated particle therapy center. An initiative since 2021 has been started by CERN Baltic Group on a novel particle therapy center development in the region in partnership with CERN NIMMS collaboration. With a conceptual design idea in early 2022 and stakeholder engagement activities in late 2022 - next step forward was necessary for the initiative for a more in-depth analysis.
Methods
A dedicated workshop “Particle therapy - future for the Baltic States? State-of-play, synergies and challenges” was held. The workshop was attended by medical community from the Baltics, as well as CERN technical experts and particle therapy practicing clinicians, with scientific programme split in 5 main areas of investigation.
Results
Current cancer epidemiology statistics and RT technological possibilities in the region were analyzed, with first estimates of eligible number of patients calculated. Technological development level of the proposed accelerator complex was discussed, as well the clinical needs and synnergy possibilities with the nuclear medicine field.
Conclusions
The current state and calculated first estimates presented here have shown a promising starting point, which prompts even further in-depth work – a feasibility study for development of a novel particle therapy center in the Baltic States.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background and introduction
According to data of the World Health Organization (WHO), cancer remains one of the most significant causes of death globally – accounting for nearly one in every six deaths globally in 2020 [1]. In 2022 alone, 19.98 million new cancer cases and 9.3 million cancer deaths were registered [2]. Throughout the years, various regions around the world have seen an increase in the incidence rates, with current estimates predicting an increase of almost 3 times by year 2050–58.6 million cases globally [3]. With global cancer burden expected to grow, effective cancer management strategies are to be considered in healthcare systems and novel treatment methods to be explored and researched.
Out of the three primary methods for cancer treatment – surgery, chemotherapy and radiotherapy (RT) – RT as treatment modality in course of care is beneficial and required in more than 50% of patients [4]. RT is frequently used in the treatment of the most widespread cancer types – breast, lung, colorectal, cervical and others. Despite the benefits of RT in cancer care path, the access to these technologies globally is inadequate, especially in countries categorized as low- or middle- income [4]. Even further, a specific modality of RT – particle therapy (PT), using positively charged ions instead of gamma photons in conventional therapy – has proven to be favourable in certain types of cancer. While clinical evidence base needs to be expanded further, proton therapy has already shown benefits in the reduction of normal tissue complications in selected types of cancer and carbon ion therapy –in treatment of radioresistant and hypoxic tumours [5,6,7,8]. Despite this, the access to this type of treatment globally is even more challenging due to increased costs of particle accelerator used. Currently, approximately 130 centres in the world offer PT, out of which only 13 offer the unique opportunities of carbon ion therapy [9], while many new development projects are in construction or planning stages.
Analysing access to particle therapy, the Baltic States – Lithuania, Latvia and Estonia – is one of the European regions without a dedicated proton or carbon ion therapy treatment centre (see Fig. 1.). Therefore, in 2021, a collaboration of research institutions and universities in the region – CERN Baltic Group (CBG) [10] – started dedicated and focused efforts on exploring possible particle therapy development paths in the region. As the name suggests, the main goal of CBG is about strengthening collaboration of Baltic States with the European Organization for Nuclear Research (CERN). Already from first discussions, development of a dedicated facility, not a commercial solution, was deemed more attractive for the region – providing more capabilities and research opportunities. Such a collaboration framework has already proven to be successful within the CERN PIMMS study, which resulted in CNAO and MedAustron ion therapy centres [11].
Particle therapy centres in Europe (ENLIGHT data, 2020) [12]
The initiative took the form of a dedicated working group “Advanced Particle Therapy centre for the Baltic States” within CBG in April 2022. The conceptual design idea was developed by the working group in the spring of 2022. Until the end of 2022, active engagement and discussions took place with relevant stakeholders – medical professionals involved in RT, scientific university representatives and involved political bodies. Following these events, key areas were identified that should be taken as first for further exploration and in-depth analysis: statistics and overall situation with cancer management in the region and clinical indications for PT eligibility, as well as technical aspects on proposed particle accelerator complex for such a facility and integration of another clinical area – nuclear medicine. To address and work on these areas, workshop with medical professionals from the Baltic region, CERN technical experts and PT practicing clinical representative from CNAO was held on May 25th, 2023 at CERN - “Particle therapy - future for the Baltic States? State-of-play, synergies and challenges”.
The aim of this work is to present key findings and points made during the workshop, as well to indicate overall conclusions and future outlooks of the initiative.
2 Overview of current status of radiotherapy technologies in the Baltic States
This section reports on key data presented regarding the cancer burden and RT treatment statistics within the region. Data regarding cancer statistics and access to RT technologies – both diagnostic and treatment units, were collected during participation of Baltic States in the “Access to Radiotherapy Technologies” (ART) study during 2022, held by The International Cancer Expert Corps (ICEC) organization [13]. Additional data corresponding to aspects specific to PT were collected in a tailored questionnaire to RT-practising clinical institutions within the region.
As of data from 2021 (or 2020 depending on data availability within the country), the 3 Baltic States have a total of 6.02 million inhabitants with a total of 38,031 newly registered cancer cases and 17,900 cancer causes deaths - a crude (non-age-specific) cancer incidence and mortality rate on average for region being 632 and 298 per 100 000 inhabitants, respectively. Country specific data are given in Table 1.
According to data collected for the year of 2020, a total of 13 045 patients within the 3 countries received RT (both external beam and brachytherapy) as part of their cancer treatment course – 6343, 4146 and 2556 for Lithuania, Latvia and Estonia, respectively. RT in the Baltic States is delivered with state-of-the-art linear accelerators − 27 in total for the region. Almost all the units are capable of delivering modern RT techniques – intensity modulation (IMRT), volumetrically modulated arcs (VMAT), as well as the high precision stereotactic techniques (SRS, SRT, SBRT) and incorporating image guidance in therapy (IGRT). The number of linear accelerator for RT for the given population can be deemed sufficient, in accordance with international guidelines (4 units per 1 million) [14], [15]. Data regarding medical personnel working in RT practice was also collected – a total of 86 radiation oncologists, 129 radiation therapy technologists (RTT) and 67 medical physicists in the 3 countries as of 2021.
Additionally, more in-depth data were also collected, such as percentage of incidence and mortality for certain cancer types and cancer localizations typically treated with protons or carbon ions (paediatrics, brain tumours, head and neck region and others). Cancer types with the highest incidence rate follow the global trends [2]: prostate, non-melanoma skin cancer, lung and breast cancer (see Table 2). Similarly, the trends are also followed for highest mortality rate: lung, colorectal, stomach and liver.
Exploring indications specific for particle therapy, more in-depth analysis was done regarding paediatric cancers. Over the period 2018–2022, a total of about 1000 paediatric cancer cases have been registered in the 3 countries, out of which about 1/5 (211 patients) have received RT as part of their treatment course. 41 of these patients were treated in the last reported year – 2022, with the most common indications being leukaemia, central nervous system tumours and lymphoma.
3 Eligibility for particle therapy: statistics implications in Baltic States case
Though various international guidelines exist from sources such as the American Society for Radiation Oncology (ASTRO) [16], as well as the healthcare systems of the United Kingdom [17] and Japan [18], overall, the most common indications for particle therapy in treatment centres are central nervous system (CNS), skull base, head and neck, and paranasal sinus tumours [5,6,7,8]. Clinical experience was shared from The National Centre for Oncological Hadrontherapy by Dr. Anna Maria Camarda, outlining clinical indications with the highest benefit and existing clinical evidence - skull base chordoma, chondrosarcoma, sinonasal carcinoma, brain tumours, head and neck tumours, radioresistant tumours and others [19]. For future perspectives, particle therapy could also provide clinical benefits in the treatment of lymphoma, lung, breast, and prostate cancers. However, a significant increase in clinical evidence is needed, as the current evidence is either conflicting, inconclusive, or lacking in general [19, 20].
In order to provide initial estimates of eligible number of cancer patients for PT, a literature review was conducted to study possible mathematical estimation approaches. Results of the literature review study are summarized in Table 3.
Based on the data provided in the Table 3 and the data collected previously – 13,045 RT receiving patients in year 2020 for all 3 countries, one can do a simple mathematical estimate:
-
based on Burnet et al. estimates [22]: around 196 patients eligible;
-
based on Glimelius et al. estimates [23]: around 1957 patient eligible.
Although this is a very simplified approach, it does provide first estimates for assessing the feasibility of PT in the region. According to the statistics of European PT centres [26], on average 223 adult patients and around 150 paediatric patients are treated per centre, as per data of 2020. First estimates do suggest that the number of PT eligible patients from the Baltic States might be sufficient for such a facility. Though, more in-depth analysis should be done in the future based on cancer incidence and RT practice for different cancer types in the clinics within the Baltic States. This is a currently on-going work and to be extended even further.
It should be noted, due to lacking clinical evidence in particular cancer types, throughout the years alternative methods have been developed for patient selection for PT. Such examples are cost-effectiveness assessment, dosimetric comparison and recently emerging normal tissue complication probability (NTCP) modelling. The latter approach proves to be a beneficial estimation tool in head and neck tumours, with development efforts for algorithms as well in brain, breast and other types of cancer [27,28,29]. As these tools would be highly beneficial in the case of the Baltic States, the necessity of modern cancer registries becomes of uttermost importance.
4 A novel path – helium ion therapy
From the technical perspective, the core technology considered for development of such a facility is the helium synchrotron – a compact medical synchrotron in active development by the Next Ion Medical Machine Study (NIMMS) collaboration [30] at CERN. The choice of helium-4 ions as the design particle for the machine has been made to address the recent re-emergence of interest in application of this ion type for cancer therapy. A clear research interest can be seen in ion therapy centres both in Europe and Asia [31,32,33]. As the role of helium ion therapy for cancer treatment is yet to be explored, particle accelerator systems for helium ion therapy would be highly beneficial to allow the necessary clinical research.
From clinical perspective, use of helium-4 ions for cancer therapy was already explored in the early stages of PT back at Lawrence Berkley National Laboratory [34], with the current renaissance mainly emerging from Heidelberg Ion Therapy centre, with the first patient treated in 2022 [31]. From a physical perspective, use of helium ions compared to protons could greatly increase the dose conformality due to reduced range straggling and lateral scattering (see Fig. 2.) and also increase the biological effectiveness. While in comparison to carbon ion beams, helium provided reduced fragmentation tail and more importantly - smaller and less demanding accelerator system would be necessary. Early treatment plan modelling studies have indeed shown helium-4 ions as a possible evolution of proton therapy, reducing the normal tissue toxicity in certain clinical scenarios [35,36,37].
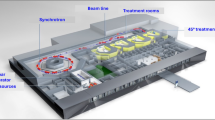
One of the main design considerations for the development of this accelerator is also to reduce the footprint of the facility and the cost, compared to carbon ion therapy facilities. The technology under development is a compact normal conducting (1.65 Tesla magnets) synchrotron with an estimated footprint of about 2200 m2 [38]. The system is designed for acceleration of fully stripped helium-4 ions with treatment relevant energies up to 220 MeV/u, with the possibility of proton acceleration, as well, correspondingly to energies of about 700 MeV, thus usable for full-body radiography applications and research. A flexible extraction system is foreseen, able to deliver ultra-high dose rates suitable for the novel FLASH therapy. The linear accelerator injector system could also provide novel dual functionality, being able to produce radioisotopes for nuclear medicine. A schematic representation of the preliminary design of a facility incorporating the proposed accelerator is given in Fig. 3. In the preliminary design of the facility two treatment rooms are foreseen, with a dedicated beam-line for research, though possible adaptations can be considered in further development stages of the initiative.
Although the design particle of the machine is helium-4 ion, the synchrotron could also deliver clinically established proton therapy as for helium-4 ion usage the process of clinical trials is yet to start. Adopting such a design for a clinical facility allows more customizability and opportunities for research and skill development of the personnel. Most of the components necessary for the technology are rather standard, with additional R&D mainly required for FLASH delivery: beam extraction, beam delivery system and delivery method itself, as well as dosimetry, beam monitoring and other safety systems. With these unique opportunities, such a facility would allow development of a vast program both in clinical domain and scientific research.
5 Beyond particle therapy – possible integration of nuclear medicine
Although the core function of the accelerator complex is the use in particle therapy, as mentioned, the dual function linear accelerator will also allow parallel production of radioisotopes for nuclear medicine. The usage of a linear accelerator would allow more efficient production with deuteron and alpha particle beams compared to cyclotrons due to increased beam transmission [38, 39]. Production of radioisotopes would be completely independent from the ion therapy and scientific research functions, as it would be done with additional beam pulses in the linear accelerator structure only. Operation mode for the synchrotron is foreseen at 1 Hz, while for the linear accelerator – at 50 Hz. As the linear accelerator can be modulated on pulse-to-pulse basis, the beam can be independently adapted for the different functions of the facility [38, 39].
While various radioactive isotopes for production have been considered from the technical possibility perspective, survey data from clinical users were presented within the framework of the PRISMAP Consortium [40,41,42]. With a total of 114 respondents from 30 European countries and 104 different institutions (out of which 48 respondents from research institutions and 40 clinical institutions) the main interests and demands for the future in nuclear medicine are for theragnostic and targeted alpha therapy isotopes – actinium-225 and other alphas emitters, copper-64 and isotopes from scandium and terbium families. Possible use of such isotopes would also be a novelty for the Baltic States, as currently only more conventional isotopes are used such as fluorine-18, technetium-99m, iodine-123 and iodine-131, lutetium-177, radium-223.
Integrating these clinical interests into the technical design of the facility is highly important. As production of non-conventional isotopes could be done in the proposed facility, possible export pathways should be considered in co-operation with the 2 soon-operational cyclotron production facilities in Lithuania and Latvia [43][44].
5.1 Findings of the workshop. Future outlooks
Development of a particle therapy centre within the Baltic States based on NIMMS helium synchrotron technology is a unique opportunity for the region to evolve both in clinical and scientific research capacity. From the technological point of view, the accelerator complex provides customizability to user needs, vast research spectrum possibilities, while keeping R&D risk minimal owing to standard technology usage in the design. The customizability also corresponds to the envisioned usage of such a facility – both as a scientific research centre and a clinical treatment facility. One of the key considerations before further developments was, of course, whether the number of patients eligible for particle therapy would be sufficient to run such a facility. The first estimates presented here have shown a promising starting point, which prompts for more in-depth analysis of this aspect in the future.
An important aspect regarding the availability of cancer statistics data for such an initiative was also put forward. For long-term goals of this initiative, development strategies are needed to provide state-of-the-art national cancer registries. Improvements can be considered for the existing registries in Lithuania and Estonia, though this aspect is even more important in Latvia, as currently a dedicated registry is lacking, which already complicated some of the data collection procedures. A consensus within the workshop was reached that the creation and improvement of national cancer registries are crucial for the success of such a proposed facility, as this data is necessary to make joint decisions between the 3 Baltic States on the number of eligible patients, as well as patient referral and reimbursement system functioning. From a clinical perspective, strengthening the support of the Baltic medical community for this initiative is crucial. A long-term project of this scale cannot be planned without clear and direct support from the medical communities of the region.
Throughout the workshop, the importance of scientific research function was discussed heavily, as well. As the helium synchrotron would be a custom-made particle accelerator, the scientific research function of the proposed facility is of high importance, with a broad programme to be foreseen. Pre-clinical and clinical research will be of high importance to develop the role of helium ion therapy in cancer treatment. The facility would also provide research opportunities in medical physics, dosimetry, accelerator physics, and related technology development, while the use of the linear accelerator for radioisotope production – in nuclear medicine, nuclear physics, radiochemistry, material science, and others. The proposed facility has a large scientific research potential, thus a more detailed programme is to be developed in the future within the foreseen feasibility study, as discussed next.
Findings of the workshop have gathered support both from medical communities and political bodies within the Baltic States for further investigations of the feasibility of such a facility. Such investigations are planned to be carried out in a dedicated longer-term feasibility study done by Baltic States specialists and researchers in close collaboration with CERN experts. The length of the feasibility study is envisioned to be 2 years, with the finalization of the programme and working plan currently on-going. The feasibility study is to focus on 3 main areas: cancer epidemiology in the Baltic States and clinical aspects, technical integration of the helium synchrotron into a dedicated facility and lastly – economic aspects.
Data availability
Not applicable.
Code availability
Not applicable.
References
Cancer. accessible online: https://www.who.int/news-room/fact-sheets/detail/cancer.
Cancer Today. accessible online: https://gco.iarc.fr/today/.
Cancer Tomorrow. accessible online: https://gco.iarc.fr/tomorrow/.
World Health Organization. Technical specifications of Radiotherapy Equipment for Cancer Treatment. World Health Organization; 2021.
Chen Z, Dominello MM, Joiner MC, Burmeister JW. Proton versus photon radiation therapy: a clinical review. Front Oncol. 2023; 13.
Mohan R. A review of proton therapy – current status and future directions. Precision Radiation Oncol. 2022;6(2):164–76.
Mohamad O, Yamada S, Durante M. Clinical indications for Carbon Ion Radiotherapy. Clin Oncol. 2018;30(5):317–29.
Malouff TD, Mahajan A, Krishnan S, Beltran C, Seneviratne DS, Trifiletti DM. Carbon Ion Therapy: a modern review of an Emerging Technology. Front Oncol. 2020; 10.
PTCOG - Facilities in Operation. accessible online: https://www.ptcog.site/index.php/facilities-in-operation-public.
CERN Baltic Group. · Indico, accessible online: https://indico.cern.ch/category/10023/.
Bryant PJ, Badano L, Benedikt M, et al. Progress of the Proton-Ion Medical Machine Study (PIMMS). Strahlenther Onkol. 1999;175(S2):1–4.
Home | THE EUROPEAN NETWORK FOR LIGHT ION HADRON THERAPY. accessible online: https://enlight.web.cern.ch/.
ART (Access to Radiotherapy Technologies) Study. - ICEC, accessible online: https://www.iceccancer.org/artstudy/.
IAEA (International Atomic Energy Agency) (2011). Planning national radiotherapy services: a practical tool. IAEA Human Health Series No.14. Vienna, Austria: International Atomic Energy Agency.[15] IAEA (International Atomic Energy Agency), Slotman BJ, Cottier B, Bentzen SM, Heeren G, Lievens Y, van den Bogaert W. Overview of national guidelines for infrastructure and staffing of radiotherapy. ESTRO-QUARTS: Work package 1. Radiotherapy and Oncology 2005; 75(3): 349.E1-349.E6.
Slotman BJ, Cottier B, Bentzen SM, Heeren G, Lievens Y, van den Bogaert W. Overview of national guidelines for infrastructure and staffing of radiotherapy. ESTRO-QUARTS: Work package 1. Radiotherapy and Oncology 2005; 75(3): 349.E1–349.E6.
ASTRO Proton Beam Therapy Model Policy. accesible online: https://www.astro.org/ASTRO/media/ASTRO/Daily%20Practice/PDFs/ASTROPBTModelPolicy.pdf.
„NHS commissioning » Proton beam therapy. accesible online: https://www.england.nhs.uk/commissioning/spec-services/highly-spec-services/pbt/.
English Translation of JASTRO treatment policy of proton beam therapy. accessible online: https://www.jastro.or.jp/en/news/proton_guideline_jastro_7_13_2017-2_cmarkandwatermark.pdf.
Camarda AM. Workshop „Particle therapy - future for the Baltic States? State-of-play, synergies and challenges session II: Clinical indications for proton and particle therapy. Existing clinical evidence and on-going clinical trials, accesible online: https://indico.cern.ch/event/1251461/contributions/5334487/attachments/2653325/4594489/Camarda_Clinical%20indications%20for%20particle%20therapy.pdf.
Mohan R, Grosshans D. Proton therapy - Present and future. Adv Drug Deliv Rev. 2017;109:26–44.
Ebner D, Malouff T, Waddle M, Foote R. HSR22-137: Proton Radiotherapy Utilization for patients diagnosed in 2018: a National Cancer Database Analysis. J Natl Compr Canc Netw. 2022;20(35):HSR22–137.
Burnet NG, Mackay RI, Smith E, Chadwick AL, Whitfield GA, et al. Proton Beam therapy: perspectives on the national health service England clinical service and research programme. Br J Radiol. 2020;93(1107):20190873.
Glimelius B, Ask A, Bjelkengren G, et al. Number of patients potentially eligible for proton therapy. Acta Oncol. 2005;44(8):836–49.
Burnet NG, Mee T, Gaito S et al. Estimating the percentage of patients who might benefit from proton beam therapy instead of X-ray radiotherapy. BJR. 2022; 95(1133).
Lee SU, Yang K, Moon SH, Suh Y-G, Yoo GS. Patterns of Proton Beam Therapy Use in Clinical practice between 2007 and 2019 in Korea. Cancer Res Treat. 2021;53(4):935–43.
Tambas M, van der Laan HP, Steenbakkers RJHM, et al. Current practice in proton therapy delivery in adult cancer patients across Europe. Radiother Oncol. 2022;167:7–13.
Tambas M, Steenbakkers RJHM, van der Laan HP, et al. First experience with modelbased selection of head and neck cancer patients for proton therapy. Radiother Oncol. 2020;151:206–13.
Dutz A, Lühr A, Troost EGC, et al. Identification of patient benefit from proton beam therapy in brain tumour patients based on dosimetric and NTCP analyses. Radiother Oncol. 2021;160:69–77.
Langendijk JA, Lambin P, De Ruysscher D, Widder J, Bos M, Verheij M. Selection of patients for radiotherapy with protons aiming at reduction of side effects: the model-based approach. Radiother Oncol. 2013;107(3):267–73.
NIMMS | Knowledge Transfer. accesible online: https://kt.cern/medtech/nimms.
Tessonnier T, Ecker S, Besuglow J, et al. Commissioning of Helium Ion Therapy and the first patient treatment with active Beam Delivery. International Journal of Radiation Oncology Biology Physics; 2023.
Gambino N, Kausel M, Guidoboni G, et al. First injector commissioning results with Helium Beam at MedAustron. Ion Therapy Centre J Phys : Conf Ser. 2022;2244(1):012109.
Quantum Scalpel Project 2021 National Centre of Radiological Sciences (NIRS). accesible online: https://www.qst.go.jp/site/innovative-project-english/quantum-scalpel.html.
Saunders W, Castro JR, Chen GTY, et al. Helium-Ion Radiation Therapy at the Lawrence Berkeley Laboratory: recent results of a Northern California Oncology Group Clinical Trial. Radiat Res. 1985;104(2):S227.
Tessonnier T, Mairani A, Chen W et al. Proton and Helium ion radiotherapy for meningioma tumors: a Monte Carlo-based treatment planning comparison. Radiat Oncol 2018; 13(1).
Wickert R, Tessonnier T, Deng M, et al. Radiotherapy with Helium ions has the potential to Improve both Endocrine and Neurocognitive Outcome in Pediatric patients with Ependymoma. Cancers. 2022;14(23):5865.
Bonaccorsi SG, Tessonnier T, Hoeltgen L, et al. Exploring helium ions’ potential for Post-mastectomy Left-sided breast Cancer Radiotherapy. Cancers. 2024;16(2):410.
Vretenar M, Angoletta ME, Benedetto E et al. A Compact Synchrotron for Advanced Cancer Therapy with Helium and Proton Beams. Proceedings of the 13th International Particle Accelerator Conference. 2022; IPAC2022: Thailand.
Vretenar M, Mamaras A, Bisoffi G, Foka P. Production of radioisotopes for cancer imaging and treatment with compact linear accelerators. J Phys : Conf Ser. 2023;2420(1):012104.
Project description, accesible online. https://www.prismap.eu/about/project/.
Radzina M, Mamis E, Saule L et al. Deliverable 5.1 - Questionnaire on industrial and clinical key players and needs. 2022, accesible online: https://zenodo.org/records/7154340.
Radzina M, Saule L, Mamis E et al. Novel radionuclides for use in Nuclear Medicine in Europe: where do we stand and where do we go? EJNMMI Radiopharm chem. 2023; 8(1).
State-of-the. -art nuclear medicine centre opens in Riga - Labs of Latvia, accesible online: https://labsoflatvia.com/en/news/state-of-the-art-nuclear-medicine-centre-opens-in-riga.
One of the most expensive purchases of the health system reached Lithuania. - a cyclotron - LRT, accesible online: https://www.lrt.lt/naujienos/sveikata/682/2009504/lietuva-pasieke-vienas-brangiausiu-sveikatos-sistemos-pirkiniu-ciklotronas?utm_source=ground.news&utm_medium=referral
Acknowledgements
We want to express our gratitude to all the speakers and moderators of the workshop, included as co-authors of this work – without them such fruithful results would not be reached!
Funding
This work has been partly funded by Latvian State Research programme VPP-IZM-CERN-2022/1–0001 and partly funded by the European Union’s Horizon 2020 research and innovation program under grant agreement No 101008548 (HITRIplus).
Open access funding provided by CERN (European Organization for Nuclear Research)
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception, design and data acquisition provided in the study. The first draft of the manuscript was written by Kristaps Paļskis, with corrections done by Erika Korobeinikova, Manjit Dosanjh, Maurizio Vretenar and Toms Torims. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Paļskis, K., Korobeinikova, E., Bogorada-Saukuma, D. et al. “Particle therapy - future for the Baltic states?” – synthesis of the expert workshop report. Health Technol. (2024). https://doi.org/10.1007/s12553-024-00875-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12553-024-00875-2