Abstract
Background
The general practice for all radiographic procedures is to use Lead Shielding to optimise radiation dose to the patient. The American Association of Physicists (AAPM) in Medicine in 2019 however, made a recommendation on the use of gonad and foetal shielding. The authors have noticed that very few papers on this topic come from developing countries, hence this study embarked on. The aim of our study was to evaluate internal scatter with Lead and without Lead Shielding in an anthropomorphic phantom during Computed Tomography of the brain, chest, abdomen, and pelvis.
Methods
The methodology was based on examinations of a RANDO phantom brain, chest, abdomen and pelvis on a General Electric Optima 660 scanner; which had a 128-channel multidetector row. Examinations were performed with Lead and without Lead Shielding equivalence of 0.35 mm, and the internal scatter measured using Thermoluminescent Dosimeters. The collected data was analysed descriptively to determine the mean and standard deviation. The T-tests and two-way analysis of variance (ANOVA) were used to compare the means.
Results
The findings of this study revealed that internal scatter was highest closest to the exposed area with higher internal scatter observed for thicker areas of the phantom. Although slightly higher readings were recorded without Pb shielding, a non-statistical significance was observed for all internal scatter measurements regardless of whether Lead Shielding was used or not.
Conclusions
A non-statistical significance for Computed Tomography examinations with and without Lead Shielding in confirmation with the AAPM positional statement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Computed Tomography (CT) was first discovered in 1972 by Sir Godfrey Hounsfield [1]. Since then, CT has developed into an extensively utilised diagnostic imaging technique. The majority of CT examination are sought for “routine” head and body applications, but the tests that are less common and need more technical expertise are the ones that garner the greatest attention for CT technology [2, 3]. Computed Tomography offers multi-slice helical imaging of a large volume of body tissue and, it is used for screening, therapeutic and a variety of diagnostic purposes [4]. It is widely acknowledged as a valuable diagnostic imaging modality that produces high-quality, detailed, cross-sectional images [5]. Computed Tomography examinations account for an estimated 44% of the global increase in radiation dose [5, 6]. While CT imaging offers valuable benefits, there is a growing concern that it is associated with an increased risk of biological effects and cancer due to increased radiation exposure. The CT Scanner manufacturers are therefore compelled by concerns about radiation risks to lower radiation doses [7].
The International Commission on Radiological Protection (ICRP) is an organisation that publishes rules and recommendations for the use of radiation globally. These recommendations have always been based on the latest scientific data and incorporates additional value judgments based on societal, economic, ethical and application experience. One such recommendation is that occupational exposure for ionising radiation should not result in an effective dose greater than 20 mSv annually, averaged over five year intervals (100 mSv in 5 years), and 50 mSv in any one year [8,9,10]. Patients are also considered for the risk of radiation. In 1905 gonadal shielding was introduced for patients and in 1907 shielding of testes was recommended for therapeutic and diagnostic X-ray exposure [11]. The general practice for all radiographic procedures was the use of Lead (Pb) shielding to reduce radiation dose to patients [11, 12]. However, these general practices changed when a positional statement was released by the American Association of Physicists in Medicine (AAPM). This statement indicated that the benefit of using gonad and foetal shielding are minimal to non-existent. When gonad and foetal shielding are positioned in the area of interest, they have the potential to obscure pathological findings or essential anatomical structures. In addition, if such shielding falls within the ionising chamber field during the use of automatic exposure controls, it may increase radiation exposure for the patient [13]. There is a scarcity of research that is devoted to investigating internal scatter and the use of Pb Shielding in CT, especially in the case of developing countries. Internal scatter as referred to in this paper is X-rays that propagates through the patient without interaction and/or X-rays that scatter within the patient through the Compton effect [4]. Therefore, investigating the impact of Pb Shielding on internal scatter is an important endeavour. The current study did so by evaluating internal scatter with and without Pb Shielding in an anthropomorphic phantom during CT of the brain, chest, abdomen, and pelvis.
2 Methods
This study used an anthropomorphic adult RANDO phantom [14, 15] and thermoluminescence detectors (TLDs), demonstrated in Figs. 1 and 2, to evaluate the internal scatter in the brain, chest, abdomen, and pelvis with Pb and without Pb Shielding. The RANDO phantom is made up of slabs/slices labelled 1 to 36. Selected slices were chosen for the brain, chest, abdomen, and pelvis in this study. The dimensions of the TLD chips were 4.5 mm x 0.24 mm and they were made of Lithium Fluoride doped with Magnesium and Titanium (LiF: Mg, Ti). Three TLDs were used for each measurement, with Pb and without Pb Shielding.
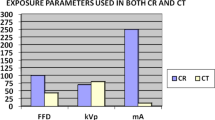
The CT scanner was a 128-channel multidetector-row General Electric Optima CT 660 and the Pb shield used was 0.35 mm Pb equivalent. Quarterly quality control tests as recommended by the South African Health Products Regulatory Authority (SAHPRA), which follow the Institute of Physics and Engineering in Medicine recommendations for routine performance testing of diagnostic X-ray imaging systems [16], were performed on both the CT scanner and Pb apron to ensure the integrity of both. Appendix A demonstrates the tests performed with the results. The scanning parameters used on the CT were pre-selected to ensure uniform exposures with Pb and without Pb Shielding and are given in Table 1.
The Pb shield was draped over the RANDO phantom, and did not fully surround the phantom during Pb Shielding measurements, as demonstrated in Fig. 3.
The exposed TLDs were read by the the Harshaw TLD Model 3500 Reader. The TLDs were then annealed using the Thermolyne 47,900 furnace, preparing them for the next examination. The calibration process followed in this study is explained in Appendix B. The collected data was analysed descriptively to determine the mean and standard deviation. The t-tests and two-way analysis of variance (ANOVA) tests were used to compare the means and Tukey post hoc analysis was performed.
Ethical conduct relates to avoiding error, promoting the aims of the research, prohibiting falsifying, fabricating, or misrepresenting research data, and promoting truth [20]. Ethical clearance for our study was granted.
3 Results
Measurements were undertaken three times. The mean TLD readings for the three slots in slices 4 (brain), 17 (chest), 24 (abdomen) and 30 (pelvis) with Pb and without Pb Shielding for the CT examinations of the brain, chest, abdomen and pelvis are presented in Table 2.
Higher internal scatter absorbed doses were observed for measurements without Pb Shielding for all areas looked at in this study. This was attributed to scattered radiation generated in the head and gantry of the CT unit which reaches the exposed area from outside and can be reduced by Pb Shielding. Also observed was higher internal scatter for thicker areas of the phantom, this was attributed to the greater electron density of the larger volume of tissue resulting in an increase in the production of scatter, which has been well documented in the literature [4, 13, 19]. Length of the scatter area was another factor that influenced the internal scatter in our study. Internal scatter decreased further away from the exposed area, with the area adjacent to the exposed area receiving the largest internal scatter and the area furthest the least internal scatter. This was observed for internal scatter measurements both with Pb and without Pb. A similar observation was made by Brnic et al., which attributed this to the intensity of external scatter decreasing proportional to the square of the distance from its source [19]. For our study this was a statistical significant decrease of internal scatter absorbed dose for the CT examinations of the brain, chest, abdomen and pelvis.
The mean difference with Pb and without Pb by two way ANOVA in conjunction with Tukey’s test is given in Table 3.
The differences in the data concerning the use of Pb and without Pb followed a normal distribution in all statistical variables. There was a statistically non-significance for all CT examinations with Pb and without Pb. Our study therefore demonstrates that whether one uses Pb Shielding or not the internal scatter will be the same, and cannot be reduced with the use of Pb Shielding. This is in agreement with the AAPM positional statement on Pb Shielding [13].
4 Discussion
Exposure outside the area of the primary beam occurs from internal scatter. We demonstrated that using Pb Shielding in CT was statistically non-significant in minimising internal scatter. There was however a 2.0% reduction recorded in data with Pb Shielding, which was attributed to the absorption of scattered radiation from the head and gantry of the CT unit. Similarly, a study assessing the application of Pb Shielding for chest CT examination observed a reduction in the dose outside of the scan field, although the reduction was minimal [17]. Breast skin dose reductions were reported when Pb Shielding was employed for CT examinations of the brain, abdomen-pelvis, liver dynamic, lumbar spine, and neck. The reduction in breast skin dose with the use of Pb Shielding, due to its proximity to the exposed area and the absorption of scattered radiation from the head and gantry of the CT unit is widely recommended and reported in literature [19, 20].
This study demonstrated that internal scatter levels measured with Pb and without Pb Shielding was higher closest to the exposed area and decreased distant from it. Chung et al. [18] confirmed an inverse square law relation during CT when entrance surface doses were investigated. The entrance surface doses closest to the primary radiated area were greater than those in distant areas. The geometry of radiation is particularly important in determining the scatter exposure of an organ. It is essential to emphasise that internal scatter is the primary contributor to the radiation dose to radiosensitive tissues. The exposure to CT radiation from the primary beam is influenced by scatter radiation from the head and gantry, spreading to other secondary areas. The intensity of external scatter decreases as the distance from its source increases. Additionally, the larger the volume of tissue, the more internal scatter occurs. Most of the exposure outside the primary beam area comes from internal scatter. It is essential to note that external shielding does not reduce internal scatter. Internal scatter is a significant source of radiation exposure for sensitive tissues, particularly those deep within the body. Therefore, shielding is less effective when the scatter source is within a patient’s body.
The levels of internal scatter for the CT abdomen differ when Pb Shielding is applied, compared to when Pb shielding is absent. However, the abdomen contains a peritoneal cavity consisting of soft tissue organs, with only the spine providing a bony structure. Hsieh et al. [21] showed that the effective doses of kidney-ureter-bladder, intravenous urography and abdominal CT were 0.22, 1.51, and 8.21–9.27 mSv respectively where the CT yielded the highest effective dose. The chest, on the other hand, is filled with air due to the presence of lungs and has a thinner bony structure from the ribs. Therefore, there is minimal attenuation, and only a low radiation-absorbed dose is received. The brain has a small area of view and thinner cranial bones, requiring less dose for penetration.
A limitation of our study was the use anthropomorphic phantom to study internal scatter, as it does not fully replicate the radiation scatter that can occur in actual human bodies with organic tissues. The phantom also simulates an average human size, but skinny and obese patients may yield different radiation dose estimates. Another limitation is the use of only 0.35 mm equivalent Pb and no other thicknesses. However, the authors believe that the internal scatter reduction and no difference with Pb and without Pb Shielding will still be seen with actual human tissue and other Pb equivalent thicknesses, hence the use of the RANDO phantom and 0.35 mm equivalent Pb was acceptable.
5 Conclusion
This study wanted to investigate internal scatter in CT examinations using an anthropomorphic phantom. The study’s findings revealed that areas that were distant from the exposed area had lower internal scatter levels than those closer to it. The internal scatter levels throughout the CT examinations of the brain, chest, abdomen and pelvis exhibited no significant difference, regardless of whether Pb Shielding was used or not. This was in agreement with the AAPM positional statement. The internal scatter, was however dependent on the thickness of the RANDO phantom.
Data availability
The authors make this data available to the journal.
Code availability
No codes were used in the study.
References
Fleischmann D, Boas FE. Computed tomography-old ideas and new technology. Eur Radiol. 2011;21(3):510–7.
Hsieh J, Flohr T. Computed tomography recent history and future perspectives. J Med Imaging. 2021;8(05):1–24.
Schulz RA, Stein JA, Pelc NJ. How CT happened: the early development of medical computed tomography. J Med Imaging. 2021;8(05).
Bushong S. Radiologic science for technologists. 11th edition. St Louis, MO: Elsevier. 2017.
Ozsahin DU, Uzun B, Musa MS, Ozsahin I. Evaluating X-ray based medical imaging devices with fuzzy preference ranking organisation method for enrichment evaluations. Int J Adv Comput Sci Appl. 2018;9(3):7–10.
Brendlin AS, Winkelmann MT, Do PL, Schwarze V, Peisen F, Almansour H, Bongers MN, Artzner CP, Weiss J, Kim JH, Othman AE, Afat S. Simulated radiation dose reduction in whole-body CT on a 3rd generation dual-source scanner: an intraindividual comparison. Diagnostics. 2021;11:118.
Rehani MM. Patients undergoing recurrent CT scans: assessing the magnitude biological effects of ionizing radiation board. 2020;1828–36.
Applegate KE, Rühm W, Wojcik A, Bourguignon M, Brenner A, Hamasaki K, Imai T, Imaizumi M, Imaoka T, Kakinuma T, Nishimura N, Okonogi N, Ozasa K, Rube CE, Sadakane A, Sakata R, Shimada Y, Yoshida K, Bouffler S. Individual response of humans to ionising radiation: governing factors and importance for radiological protection. Radiat Environ Biophys. 2020;59(2):185–209.
Pearson DD, Provencher L, Brownlee PM, Goodarzi AA. Modern sources of environmental ionizing radiation exposure and associated health consequences. 2nd ed. Genome Stability: From Virus to Human Application. Elsevier Inc. 2021:603–619.
Paduka S, Thongsawad S, Janthawanno P, krikaew K, Khaeongrod R, Ketphan K, Saiyo N. Assessment of organ doses from head and neck cone-beam computed tomography (CBCT) in adaptive radiation therapy: a phantom study. Radiat Phys Chem. 2024;21.
Jeukens CRLPN, Kütterer G, Kicken PJ, Frantzen MJ, van Engelshoven JMA, Wildberger JE, Kemerink GJ. Gonad shielding in pelvic radiography: modern optimised X-ray systems might allow its discontinuation. Insights into Imaging. 2021:11(1).
Hayre CM, Bungay H, Jeffery C. How effective are lead-rubber aprons in protecting radiosensitive organs from secondary ionising radiation? Radiography. 2021:26(4).
American Association of Physicists in Medicine (AAPM). AAPM position statement on the use of patient gonadal and fetal shielding. Available from: AAPM (American Association of Physicists in Medicine). 2019).
Rodrigues F, Nolasco A, Meira Belo LC, Silva C, Fonseca T. Assessment of dose heterogeneity in TBI using the thorax of the anthropomorphic Alderson-Rando phantom and TLDs in two different setups. Brazilian J Radiat Sci. 2023;11(1A):01–13.
Paduka S, Thongsawad S, Janthawanno P, krikaew K, Khaeongrod R, Ketphan K et al. Assessment of organ doses from head and neck cone-beam computed tomography (CBCT) in adaptive radiation therapy: A phantom study. Radiat Phys Chem. 2024;215(October 2023):111338. https://doi.org/10.1016/j.radphyschem.2023.111338
IPEM (Institute of Physics and Engineering in. Medicine) recommended standards for the routine performance testing of diagnostic X-ray imaging systems, Report no. 91. 2005.
Yu L, Bruesewitz MR, Vrieze TJ, McCollough CH. Lead shielding in pediatric chest CT: effect of apron placement outside the scan volume on radiation dose reduction. AJR Am J Roentgenol. 2019;212(1):151–6.
Chung JJ, Cho ES, Kang SM, Yu JS, Kim DJ, Kim JH. Usefulness of a lead shielding device for reducing the radiation dose to tissues outside the primary beams during CT. Radiol Med. 2014;119(12):951–7.
Brnić Z, Vekić B, Hebrang A, Anić P. Efficacy of breast shielding during CT of the head. Eur Radiol. 2003;13(11):2436–40.
Zalokar N, Mekis N. Efficacy of breast shielding during head computed tomography examination. Radiol Oncol. 2020;55(1):116–20.
Hsieh TY, Chen SL, Chang YR, Tyan YS, Chen TR. Effective dose for kidney-ureter-bladder plain radiography, intravenous urography, and abdominal computed tomography scan: a phantom study. Appl Radiat Isot. 2022:18.
Acknowledgements
The authors would like to express their sincere gratitude to the Nelson Mandela Children’s Hospital for the use of their CT scanner.
Funding
The authors declare that there was no funding provided for the study.
Open access funding provided by Sefako Makgatho Health Sciences University.
Author information
Authors and Affiliations
Contributions
Bronwin Van Wyk established methods and analysed results; Zanele Eunice Ngobese collected data and Shantel Lewis interpreted and supervised the manuscript findings. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
University of Johannesburg Faculty of Health Sciences Research Ethics Committee with the number HDC-01-85-2021.
Consent to participate
Not applicable.
Consent for publication
All authors consent to the publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix A: example of the SAHPRA QC performed on the CT scanner
III.1.1. (2): Lead Aprons.
Lead Apron | Result |
|---|---|
Visual inspection | Pass |
III.1.12. (47) & (48): Noise & CT number values.
Water | Perspex | |
|---|---|---|
Mean | 2.57 | 130.41 |
Tolerance | 2.31–2.83 | 117.37–143.45 |
Standard Deviation | 3.89 | 4.94 |
Tolerance | 3.50–4.28 | 4.45–5.43 |
Result | Pass | Pass |
Appendix B: TLD calibration
The TLD chips were placed on the isocentric position of the X-ray bed at a source to surface distance of (SSD) of 100 cm and a field size 10 × 10 cm2. The TLDs were annealed to remove residual effects, irradiated using X-ray diagnostic energies and then read on the Harshaw 3500 system. The annealing process was performed by exposing the lithium fluoride to a temperature of 400 degrees Celsius (°C) for an hour and with a pre-read period of five minutes. The irradiation of the TLDs was performed in conjunction with the X-ray qualities according to the ISO 4047 standards offered at National Institute of Standards and Technology (NIST). A DELRINR ionization chamber with the reference conditions from a South African primary standardization laboratory was then used. The chamber had a high voltage of 1500 V, 20 °C air temperature, 760 mm Hg air pressure and 40–60% reference humidity was then irradiated under the same conditions as the TLDs. From the glow curves provided by the Harshaw 3500 system, the equation of the line was determined which in turn produced the calibration factor (CF). The CF for each TLD was obtained by dividing the response of the TLD by its corresponding dose. This factor was compared to the chambers calibration factor in Gy/Coulomb for each quality with the corresponding dose reading.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Van Wyk, B., Ngobese, Z. & Lewis, S. An investigation of internal scatter during computed tomography using an anthropomorphic phantom. Health Technol. 14, 747–752 (2024). https://doi.org/10.1007/s12553-024-00868-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12553-024-00868-1