Abstract
Purpose
Since the pioneering use of planar X-ray imaging in early experimental sites of proton and light ion cancer therapy, imaging has always been a cornerstone of ion beam therapy (IBT). This contribution highlights current trends and future perspectives of imaging in modern IBT.
Methods
Several flavours of image guidance are under investigation to enhance IBT. A first class of in-room imaging techniques aims at providing insights on updated patient anatomy prior to or ideally during treatment. Owing to the unique characteristics of IBT, these methods do not only target a correct localization of the tumour and critical structures as in photon therapy, but also aim at extracting the tissue stopping properties for accurate (re)planning. A second class of techniques, predominantly performed during beam delivery, aims at capturing different secondary emissions induced by the irradiation to identify the beam stopping position and ideally reconstruct the dose delivery for inter- or intra-fractional treatment adaptation. Finally, a third class of imaging techniques is being explored to provide novel insights on the underlying biological mechanisms to open new opportunities for more effective and better tolerated treatments.
Results and conclusions
70 years after the worldwide first proton treatment, image guidance of IBT continues to be an evolving area which combines advanced instrumentation with progress in computational areas, including artificial intelligence, and beam delivery schemes. Especially on-site imaging opens new opportunities to innovate the IBT chain with daily treatment adaptation, real-time verification of in-vivo range and dose delivery along with biological guidance for treatment personalization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The increased physical selectivity of ion beam therapy (IBT) makes it more sensitive than photon therapy to changes of the actual treatment situation with respect to the planned one. Especially the finite ion beam range in tissue, correlated to the position of the maximum dose deposition in the Bragg peak, is strongly influenced by the daily patient anatomy and physical properties of the traversed tissue. Hence, since the beginning of IBT, in-room image guidance solutions have been explored to improve tumour target conformity and organs at risk (OARs) sparing. But while the initial solutions were limited to X-ray planar imaging [1], recent upgrades of the facilities have seen the progressive introduction of in-room volumetric imaging systems able to acquire three-dimensional (3D) and even 4D information of the patient anatomy prior to treatment [2]. In comparison to photon therapy, a major focus of IBT is on imaging techniques that can provide not only updated information of the patient anatomy, but also the physical stopping power properties of the tissue to be traversed by the beam [2]. Moreover, in addition to an improved representation of the patient in the actual treatment situation, ideally continuously during the treatment itself [3], several efforts are ongoing in the field of in-vivo range and dose monitoring. Besides the solutions already envisioned in the early days of IBT aiming to visualize the β+-activity either directly implanted by radioactive ion beams or produced as a by-product of the therapeutic ion irradiation, different strategies are being pursued to exploit almost instantaneous secondary emissions to open the prospects of real-time in-vivo range verification and, potentially, reconstruction of the delivered dose [4]. Finally, although still at an early stage, several imaging techniques are being revisited or newly proposed with regard to their ability to visualize underlying biological mechanisms and features for treatment personalization (see, e.g [5]). Together with the increasing interest in new delivery schemes which can enhance the therapeutic index through temporal (e.g., “FLASH”) and spatial (e.g., minibeams) fractionation [6], this calls for an improved understanding of the physicochemical processes underlying the effects of radiation, to achieve more successful eradication of the tumour and/or improved normal tissue and OARs protection.
2 Imaging for anatomical confirmation
Horizontal as well as vertical in-room X-ray computed tomography (CT) systems on rail, and different flavours of X-ray cone beam (CB)CTs, e.g., mounted on nozzle, couch and ceiling, are entering the arena of IBT [2] to enable volumetric visualization of the patient anatomy prior to the treatment for offline or online treatment adaptation. While CT solutions offer improved diagnostics image quality for contouring and dose (re)calculation, they typically provide a “near-treatment-position” imaging, which requires some additional time and is associated with possible uncertainties when the patient is brought into the treatment position after the CT scan. On the contrary, CBCT solutions provide imaging directly in the treatment position, but result in poorer image quality due to the increased scatter fraction and less favourable imaging geometry, even more challenged by the typically larger source-to-detector distances of IBT installations with respect to those of photon therapy. Nevertheless, several approaches have been suggested for improvements of CBCT image quality [2], e.g., from iterative image reconstruction schemes to scatter correction methods based on prior CT information, deformable image registration (DIR) and artificial intelligence, benefiting also from the intense research carried out in the wider photon therapy community. And although no clear preferred strategy could be so far identified from the reported comparisons (see, e.g., Ref [7]). X-ray based imaging modalities intrinsically suffer from the drawback of additional ionizing radiation exposure and low soft tissue contrast, which limits to some extent the frequency of repeated and/or prolonged examinations (particularly in the case of planning-quality CT scans of typically higher dose than CBCTs). Especially in the challenging case of organ motion, time-resolved volumetric (4DCT) or planar (fluoroscopy) X-ray images can provide an updated dynamic model of the moving anatomy, but is associated with increased radiation dose, which again poses limitations on practical usage, especially when aiming at continuous imaging simultaneous to the dose delivery. To overcome these limitations, current explorative studies are investigating the possible integration of magnetic resonance imaging (MRI) in IBT, with already first human scale installations being integrated in experimental beamlines of pencil beam scanning delivery. In addition to solutions relying on split or C-shaped magnets with moderate field strengths of ca. 0.2 to 0.5 T [3], there are also new initiatives aiming to exploit a portable ultra-low field system (Hyperfine Inc. Swoop system) of only 0.064 T, which was found to provide reasonable radiological image quality while promising to minimize costs (no cryogens required and standard power) as well as interference with the IBT facility and dose delivery. However, all these imaging methods (single energy spectrum CT/CBCT and MRI) can only provide an improved representation of the patient anatomy for localization of relevant structures (e.g., tumour volume and OARs), without an accurate determination of the tissue stopping properties which are needed for reliable treatment plan calculations.
3 Imaging for extraction of accurate tissue stopping power ratio (relative to water)
When imaging a patient at an X-ray CT scanner with a single energy spectrum, the grey scale values of the images typically reported in terms of Hounsfield Units cannot disentangle variations due to electron density and effective atomic number, thereby resulting in an ambiguous calibration in stopping power ratios (SPR) of tissue relative to water. The same limitation applies to the standard workflow of synthetic X-ray CT generation from magnetic resonance images, as pioneered in the context of MR-guided photon therapy. Therefore, different imaging approaches are under consideration to improve the knowledge of the patient SPR for more reliable dose (re)calculations in IBT, with first solutions entering clinical application. To overcome the ambiguity inherent to the limited information provided by X-ray imaging with a single energy spectrum, one can rely on the latest innovations of diagnostic X-ray imaging, which provide so-called multi-colour imaging with different flavours of dual-energy (DE)CT implementations and even spectral imaging. The latter can exploit latest generation photon counting (PC) detector solutions able to measure the energy of individual imaging photons. The resulting additional information can be used to better disentangle the physical tissue properties, particularly the relative electron density and effective atomic number (linked to the ionization potential), which influence the stopping properties of a specific tissue with respect to water. Several investigations with tissue equivalent materials and ex-vivo tissue samples scanned at commercially available clinical DECT and PCCT scanners have shown the promise of these advanced X-ray imaging techniques to reduce the SPR estimation error from ~ 2–3% (when using standard single energy spectrum CTs) to ~ 1% (see, e.g., [8], and citations therein). These results thereby justify the recent clinical implementation of DECT-based treatment planning [9] at two proton therapy centres in Germany and US, along with attempts to integrate these advanced multi-energy X-ray imaging technologies in in-room solutions of CTs on rail and CBCTs. However, all these methods still rely on a series of calibrations and therefore offer only an indirect albeit improved estimation of SPR. A more direct measurement of tissue SPR could be deduced from imaging the patient with the same radiation quality as for treatment. This idea, also dating back to the early days of X-ray tomographic imaging and ion beam therapy, exploits the fact that the SPR has a negligible dependence on the beam energy and ion type, thus enabling the use of transmission (i.e., with sufficiently high energy to traverse the patient) proton or light ion imaging to retrieve the SPR needed for dosimetric calculations at the lower energies stopping the beam in the tumour. Several detector setups have been proposed and realized in first prototypes, often adapting particle physics instrumentation to ideally track individual ions entering/leaving the patient, and measuring their residual energy or energy loss [10]. If a complete, reasonably fast rotation of the beam or the patient is feasible, tomographic imaging could directly provide SPR maps in the treatment position for treatment (re)planning, likely offering an accuracy of SPR retrieval down to ~ 0.5-1%, according to the experimental studies reported for several phantoms and even biological tissue. Moreover, it would offer very promising possibilities of reduced radiation exposure from the intrinsic interaction properties of energetic ions in tissue and the increased flexibility of so-called fluence modulation and region-of-interest imaging in comparison to X-ray CT [11]. However, even few radiographic projections could be sufficient to refine the SPR calibration of a prior treatment planning X-ray CT, and even capture possible anatomical modifications between the planning and treatment situation for adaptive workflows [12]. Although not yet clinically implemented, first proton imaging solutions are emerging which are close to clinical testing, especially for the less cumbersome implementation of pre-treatment radiographic imaging or range probing, i.e., confirmation of the stopping position of few exploratory pencil beams going through pre-defined regions of the patient [13]. More recently, also intriguing approaches which enable refinement of SPR estimations using MR images have been proposed, leveraging the increased interest in a possible future realization of MR-guided IBT [14].
4 Imaging for visualization of the in-vivo beam range and for dose reconstruction
Despite the possibilities to combine the improved patient model (including its SPR properties) obtained from the just discussed in-room imaging solutions with actual beam records and advanced computational tools to estimate the delivered dose, it would be desirable to have means to visualize the beam stopping position in the patient in-vivo, ideally in real-time during the therapeutic treatment delivery. Hence, in-vivo beam range verification and feasibility of dose reconstruction have also been topics of active research since the very early pioneering studies of positron-emission imaging of β+-activity implanted with low dose probing radioactive ion beams, or produced as a by-product of irradiation with stable ion beams. Meanwhile, for the latter stable protons and light ion beams, different solutions of in-beam, in-room and offline positron emission tomography (PET) imaging have been investigated also in first clinical pilot trials. The reported results showed the promise but also the challenges of such an intrinsically three-dimensional imaging technique, especially due to biological washout in the time elapsed between the β+-active isotope formation and decay (with the half-lives of the major radioisotopes produced varying from 2 min for 15O to 20 min for 11C) [15]. To minimize the washout issue, latest generation in-beam PET solutions have been realized, that are capable of online (at intervals of ~ 60 s for sufficient accumulation of signal) visualization of the β+-activity building up during irradiation, for correlation to the expected distribution [16]. Moreover, additional irradiation-induced emissions have been proposed as alternative to PET imaging. Among them, the prompt gamma resulting from very fast (sub-ns) de-excitation of nuclei following nuclear interaction of ions in tissue have been regarded as the most promising signal for the monitoring of proton therapy [15]. Here, two different approaches are already entering clinical evaluation with collimated detection systems retrieving the spatial and even spectral information of these tissue-specific nuclear emissions. Additional solutions under investigation for prompt gamma monitoring either aim to enlarge the field of view of the collimator, or to achieve 3D collimator-less imaging. The latter solutions span from Compton cameras exploiting Compton kinematics of the prompt gamma interacting in multiple detector layers acting as scatterer and/or absorber, to different arrangements of very fast detectors exploiting the timing characteristics of the prompt gamma emissions, eventually even enabling their spatiotemporal emission reconstruction [17]. In these contexts, also combined detection of PET and PG emissions have been proposed. A less mature, though very intriguing alternative possible only at intrinsically (e.g., synchrocyclotron) or artificially (e.g., cyclotron) pulsed accelerators is proto- or ionoacoustics (IA) [18]. This could offer a compact and cost-effective method to monitor in real-time the Bragg peak position by multilaterating or reconstructing (depending on the number of transducers) the thermoacoustic emissions originating from the pulsed energy deposition in tissue. For suitable anatomical locations, this method also opens the prospects of real-time co-registration with the underlying patient anatomy visualized by ultrasound imaging, thereby offering a very attractive treatment monitoring option especially for anatomical sites subject to organ motion (e.g., prostate, liver, cervix). Moreover, it could become a promising option for modern delivery schemes of temporal and spatial fractionation, which are expected to naturally enhance such thermoacoustic emissions.
Despite the general issues of low signal-to-noise ratios, along with the requirements of dedicated instrumentation and computational tools that are still subject of active research and development, all introduced techniques offer possibilities of in-vivo range verification with accuracies in the order of 1–3 mm (when comparing measurements to expectations), and (sub-)mm precision when comparing inter-fractional variations. This could enable a safe reduction of margins along with the exploitation of the so far disregarded beam directions placing the steepest distal dose gradient of the Bragg peak just before OARs, thereby enabling a valuable reduction of normal tissue toxicities or dose escalation options in the tumour [19]. Those methods enabling (quasi) real-time range verification can also open the prospects of enhanced adaptive workflows during beam delivery, including the possibility of a prompt interruption of erroneous dose applications and even intra-fractional treatment plan adaptations.
In all endeavors around in-vivo monitoring techniques, the investigations have not only aimed at retrieving the beam range, ideally in real-time, but also reconstructing the delivered dose. Here, different limitations have been encountered, owing to the generally poor signal-to-noise ratio along with the intrinsic challenges of each monitoring technique, such as loss of activation-dose correlation due to biological washout in PET monitoring, limited dimensionality of collimated PG detection and uncertainties in the conversion of energy deposition to acoustic pressure in ionoacoustics. Nevertheless, progress has been reported for all these techniques in the latest years, especially thanks to advanced computational methods exploiting prior knowledge and artificial intelligence (see, e.g [20]). Moreover, new treatment planning strategies have been proposed to selectively increase the statistics of exploratory pencil beams to enable a reliable monitoring at a few locations of the treatment field, before delivering the complete plan [21].
In addition to in-vivo verification methods exploiting physical emissions induced by the irradiation, there have also been studies showing the feasibility of detecting physiological changes becoming manifest after the entire treatment in certain MR images of specific anatomical areas (e.g. spine [22], liver). And while the initial enthusiasm for this method had gradually dampened over the last decade, the new prospects of integrating MRI into image-guided IBT are reviving the interest in the possible use of MR for visualization of the beam [23] or radiation-induced tissue changes on smaller time scales during or shortly after treatment.
5 Imaging for biological guidance
While the last decades have been especially focused on harnessing imaging techniques able of controlling on-site the patient anatomy and beam delivery, the promise of personalized medicine along with the prospect of emerging new delivery modalities such as FLASH and minibeams have also shifted the focus of IBT onto biological image guidance. Here, structural and functional imaging techniques such as MRI and PET integrated in the treatment room and even in the delivery site could provide almost on a daily basis means for improved target delineation and margin definition, e.g., accounting for anisotropic tumour spread, along with a biological basis for adaptation of the dose prescription and its spatial and temporal distribution. Advances in PET imaging for sensing the mean lifetime change of positronium in dependence of the molecular environment have also been discussed to offer new promising prospects for imaging hypoxia or enable differentiation between healthy and cancerous tissue [5]. And even the PG spectroscopic techniques put forward in the context of range monitoring could enable tracking concentration variations of relevant elements such as oxygen in tissue along the treatment course, again for correlation to hypoxia or other relevant biomarkers detectable at the nuclear level [24]. Hence, the increasing adoption of advanced imaging techniques in the treatment room can pave the way to novel exploitation of information available almost on a daily basis (depending on the examination time, costs and logistics, e.g., if associated to the additional supply of contrast agents or radioactive tracers) to open new possibilities of biological image guidance in IBT.
6 Conclusion and outlook
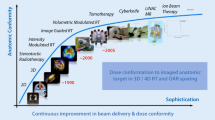
On-site imaging is becoming an essential component of the entire radiotherapy chain (Fig. 1), which can benefit from the continued advancements in dedicated instrumentation along with the new possibilities offered by improved computational power and artificial intelligence. In particular, pushing the boundaries of spatiotemporal resolution can open new prospects for anatomical image guidance, online adaptation and real-time verification of in-vivo range and dose delivery, for conventional and emerging treatment schemes in IBT. Ultimately, the improved control of the patient anatomy and dose delivery should be complemented by biological image guidance to enable an optimization and personalization of the therapy (Fig. 2), with progressive adaptation based on all information which can be collected almost on a daily basis along the course of treatment [27]. Although the latter exploitation of in-room biological information is still at its infancy, it holds great promise in combination with the new possibilities offered by recent advances in on-board (i.e., integrated in the dose delivery site) instrumentation and data processing, along with the new delivery schemes which can in turn also open new imaging opportunities (like the enhancement of thermoacoustic emissions expected in FLASH therapy [28]).
Exemplary representation of the role of advanced imaging in IBT. Although the different images do not belong to the same patient (and in the case of PET do not refer to an IBT treatment), they illustrate the role of the discussed imaging modalities in the adaptive loop of IBT. In particular, the figure demonstrates the use of advanced imaging for improved patient model for treatment (re)planning (addressed in Sects. 2 and 3), in-vivo range verification during delivery (Sect. 4) and biological assessment prior to and after treatment (Sect. 5). The type of the shown imaging modalities is highlighted in bold blue characters, and texts in brackets “()” refer to repeated operations during the treatment course, after the first treatment fraction. Data adapted from reference [7] (CT, CBCT and plan adaptation), reference [25] (expected positron emitters, PE, and prompt gamma, PG, distributions) and reference [26] (Fluorine-18 fuorodeoxyglucose PET/CT)
References
Verhey LJ, Goitein M, McNulty P, Munzenrider JE, Suit HD. Precise positioning of patients for radiation therapy. Int J Radiat Oncol Biol Phys. 1982. https://doi.org/10.1016/0360-3016(82)90530-2.
Herrick M, Penfold S, Santos A, Hickson K. A systematic review of volumetric image guidance in proton therapy. Phys Eng Sci Med. 2023. https://doi.org/10.1007/s13246-023-01294-9.
Hoffmann A, Oborn B, Moteabbed M, Yan S, Bortfeld T, Knopf A, Fuchs H, Georg D, Seco J, Spadea MF, Jäkel O, Kurz C, Parodi K. MR-guided proton therapy: a review and a preview. Radiat Oncol. 2020. https://doi.org/10.1186/s13014-020-01571-x.
Parodi K, Polf J. In vivo range verification in particle therapy. Med Phys. 2018. https://doi.org/10.1002/mp.12960.
Parodi K, Yamaya T, Moskal P. Experience and new prospects of PET imaging for ion beam therapy monitoring. Z Med Phys. 2023. https://doi.org/10.1016/j.zemedi.2022.11.001.
Mazal A, Prezado Y, Ares C, de Marzi L, Patriarca A, Miralbell R, Favaudon V. FLASH and minibeams in radiation therapy: the effect of microstructures on time and space and their potential application to protontherapy. Br J Radiol. 2020. https://doi.org/10.1259/bjr.20190807.
Nesteruk KP, Bobić M, Lalonde A, Winey BA, Lomax AJ, Paganetti H. CT-on-rails Versus In-Room CBCT for Online Daily Adaptive Proton Therapy of Head-and-Neck cancers. Cancers (Basel). 2021. https://doi.org/10.3390/cancers13235991.
Hu G, Niepel K, Risch F, Kurz C, Würl M, Kröncke T, Schwarz F, Parodi K, Landry G. Assessment of quantitative information for radiation therapy at a first-generation clinical photon-counting computed tomography scanner. Front Oncol. 2022. https://doi.org/10.3389/fonc.2022.970299.
Wohlfahrt P, Möhler C, Hietschold V, Menkel S, Greilich S, Krause M, Baumann M, Enghardt W, Richter C. Clinical implementation of dual-energy CT for Proton Treatment Planning on pseudo-monoenergetic CT scans. Int J Radiat Oncol Biol Phys. 2017. https://doi.org/10.1016/j.ijrobp.2016.10.022.
Johnson RP. Review of medical radiography and tomography with proton beams. Rep Prog Phys. 2018. https://doi.org/10.1088/1361-6633/aa8b1d.
Dedes G, Johnson RP, Pankuch M, Detrich N, Pols WMA, Rit S, Schulte RW, Parodi K, Landry G. Experimental fluence-modulated proton computed tomography by pencil beam scanning. Med Phys. 2018. https://doi.org/10.1002/mp.12989.
Palaniappan P, Meyer S, Rädler M, Kamp F, Belka C, Riboldi M, Parodi K, Gianoli C. X-ray CT adaptation based on a 2D-3D deformable image registration framework using simulated in-room proton radiographies. Phys Med Biol. 2022. https://doi.org/10.1088/1361-6560/ac4ed9.
Meijers A, Seller Oria C, Free J, Langendijk JA, Knopf AC, Both S. Technical note: first report on an in vivo range probing quality control procedure for scanned proton beam therapy in head and neck cancer patients. Med Phys. 2021. https://doi.org/10.1002/mp.14713.
Marants R, Tattenberg S, Scholey J, Kaza E, Miao X, Benkert T, Magneson O, Fischer J, Vinas L, Niepel K, Bortfeld T, Landry G, Parodi K, Verburg J, Sudhyadhom A. Validation of an MR-based multimodal method for molecular composition and proton stopping power ratio determination using ex vivo animal tissues and tissue-mimicking phantoms. Phys Med Biol. 2023. https://doi.org/10.1088/1361-6560/ace876.
Parodi K. Latest developments in in-vivo imaging for proton therapy. Br J Radiol. 2020. https://doi.org/10.1259/bjr.20190787.
Ferrero V, Fiorina E, Morrocchi M, Pennazio F, Baroni G, Battistoni G, Bisogni MG, et al. Online proton therapy monitoring: clinical test of a Silicon-Photodetector-based in-beam PET. Sci Rep. 2018. https://doi.org/10.1038/s41598-018-22325-6.
Pennazio F, Ferrero V, D’Onghia G, Garbolino S, Fiorina E, Marti Villarreal OA, Mas Milian F, Monaco V, Monti V, Patera A, Werner J, Wheadon R, Rafecas M. Proton therapy monitoring: spatiotemporal emission reconstruction with prompt gamma timing and implementation with PET detectors. Phys Med Biol. 2022. https://doi.org/10.1088/1361-6560/ac5765.
Hickling S, Xiang L, Jones KC, Parodi K, Assmann W, Avery S, Hobson M, El Naqa I. Ionizing radiation-induced acoustics for radiotherapy and diagnostic radiology applications. Med Phys. 2018. https://doi.org/10.1002/mp.12929.
Tattenberg S, Madden TM, Bortfeld T, Parodi K, Verburg J. Range uncertainty reductions in proton therapy may lead to the feasibility of novel beam arrangements which improve organ-at-risk sparing. Med Phys. 2022. https://doi.org/10.1002/mp.15644.
Liu CC, Huang HM. A deep learning approach for converting prompt gamma images to proton dose distributions: a Monte Carlo simulation study. Phys Med. 2020. https://doi.org/10.1016/j.ejmp.2019.12.006.
Tian L, Landry G, Dedes G, Pinto M, Kamp F, Belka C, Parodi K. A new treatment planning approach accounting for prompt gamma range verification and interfractional anatomical changes. Phys Med Biol. 2020. https://doi.org/10.1088/1361-6560/ab7d15.
Gensheimer MF, Yock TI, Liebsch NJ, Sharp GC, Paganetti H, Madan N, Grant PE, Bortfeld T. In vivo proton beam range verification using spine MRI changes. Int J Radiat Oncol Biol Phys. 2010. https://doi.org/10.1016/j.ijrobp.2009.11.060.
Schieferecke J, Gantz S, Hoffmann A, Pawelke J. Investigation of contrast mechanisms for MRI phase signal-based proton beam visualization in water phantoms. Magn Reson Med. 2023. https://doi.org/10.1002/mrm.29752.
Magalhaes Martins P, Dal Bello R, Ackermann B, Brons S, Hermann G, Kihm T, Seco J. PIBS: Proton and ion beam spectroscopy for in vivo measurements of oxygen, carbon, and calcium concentrations in the human body. Sci Rep. 2020. https://doi.org/10.1038/s41598-020-63215-0.
Pinto M, Kröniger K, Bauer J, Nilsson R, Traneus T, Parodi K. A filtering approach for PET and PG predictions in a proton treatment planning system. Phys Med Biol. 2020. https://doi.org/10.1088/1361-6560/ab8146.
Zhong J, Sundersingh M, Dyker K, Currie S, Vaidyanathan S, Prestwich S, Scarsbrook A. Post-treatment FDG PET-CT in head and neck carcinoma: comparative analysis of 4 qualitative interpretative criteria in a large patient cohort. Sci Rep. 2020. https://doi.org/10.1038/s41598-020-60739-3.
Ajdari A, Niyazi M, Nicolay NH, Thieke C, Jeraj R, Bortfeld T. Towards optimal stopping in radiation therapy. Radiother Oncol. 2019. https://doi.org/10.1016/j.radonc.2019.01.010.
Kim K, Pandey PK, Gonzalez G, Chen Y, Xiang L. Simulation study of protoacoustics as a real-time in-line dosimetry tool for FLASH proton therapy. Med Phys. 2023. https://doi.org/10.1002/mp.16894.
Funding
Open Access funding enabled and organized by Projekt DEAL. The author acknowledges financial support from the German Research Foundation (DFG) and the One Munich Strategy Forum around the topics of ion imaging (DFG grant number 372393016), PG monitoring (DFG grant number 441208898) and photon counting imaging (project EQAP).
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publish
Not applicable.
Competing interests
The author has no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Parodi, K. Imaging for ion beam therapy: current trends and future perspectives. Health Technol. (2024). https://doi.org/10.1007/s12553-024-00853-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12553-024-00853-8