Abstract
Purpose
Hadrons, i.e. particles that are heavier than electrons, are playing an increasingly important role in radiation oncology. Due to the high investment costs for the necessary infrastructure, this option is only available in specialized centers.
Methods
This article describes some of the physical properties that make hadrons attractive for external beam radiation therapy (EBRT), but also some of the challenges that need to be considered.
Results
The importance of linear energy transfer for biological effects is discussed.
Conclusions
In addition to the use of charged particles, the importance of neutrons for radiotherapy is also highlighted, in particular the properties of boron neutron capture therapy (BNCT), which open up completely new possibilities for the further development of EBRT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In daily clinical practice, external beam radiotherapy (EBRT) is performed using photons with energies ranging from X-rays to high mega-voltage photons up to 15 MV (even higher in some places) and electrons up to 18 MeV. Over the last 6 decades, however, dedicated centers have developed therapy modalities based on heavier particles, namely hadrons. The term “hadron” was coined by Okun [1] at the 1962 International Conference on High Energy Physics, where he called strongly interacting particles “hadrons”, as opposed to leptons, which do not undergo strong interactions. The Greek term ἁδρός means “large” or “massive” in contrast to λεπτός which signifies “small” or “light”. Radiation sources for such particles are technically challenging and expensive to purchase and operate and therefore, for a number of years, were only available in a few places worldwide. In the last 20 years, however, with the development and improvements in accelerator technology, charged particles in particular have found a permanent place in radiation oncology, whereby, at least for therapy with protons, larger hospitals specializing in tumor treatment can now provide affordable treatments. This has led to a growing interest in the radiotherapy community in therapy with hadrons in general, with the use of neutrons for tumor treatment now also attracting great interest. In this article we try to briefly explain the advantages that hadrons can have in the treatment of patients.
2 How to improve radiotherapy?
When we think about ways to improve radiotherapy, there are two basic principles that we can apply.
The first is to improve the physical selectivity, with the goal to apply a high dose to the tumor and a low dose to the surrounding normal tissues. “Precision” is the term to describe this approach, which has led to most of the progress made in radiation oncology over the last 30 years. Here is the place for technological improvements, physics, geometry, and imaging. Immense advances in computer technology, which have made it possible to evaluate and improve complex dose distributions in a volume, as well as modern imaging technologies and ingenious beam guidance systems, play an important role here. Charged particles also come into play in this approach. Sparing normal tissues is resulting in less acute and late side effects. Dose escalation in the tumor leads to improved local control with potentially higher cure rates.
On the other hand, there is the possibility to improve the differential effect of the radiation, with the goal to increase the effects on the tumor and to reduce the effects on normal tissues. Radiobiology, combined treatments with radiation and drugs, as well as the use of radiation with high linear energy transfer (LET) are the key components of this approach. In this article we concentrate on radiation quality and have to stress the latter point. Higher LET means a high density of ionization per unit distance and results an increased number of double strand breaks to the DNA that are difficult to repair. In addition, this effect is not depending on the concentration of oxygen in tissue resulting in a low oxygen enhancement ratio (OER). It increases the relative biological effectiveness (RBE) and leads to a small differential radiosensitivity between different cell lines. From a clinical point of view, with a high LET radiation a higher effectiveness can be expected in hypoxic, well-differentiated, slow-growing and radiation-resistant tumors.
3 What kind of hadrons are used for therapy?
The particles most commonly used in EBRT are negatively charged electrons, which are accelerated in conventional medical linear accelerators to be applied directly or to hit a target that generates bremsstrahlung, which is used for therapy. However, electrons are particles that are not affected by the strong nuclear forces but are only subject to electromagnetic forces and belong to the family of lepton particles. The mass of an electron is 9.109 383 7015 × 10−31 kg [2], which is about 1/1800 the mass of a proton or neutron.
Hadrons, which are currently used for cancer therapy, are charged particles such as protons and composite particles (e.g. helium ions, carbon ions) on the one hand and uncharged particles, namely neutrons, on the other (Table 1). Possible therapeutic advantages of using charged particles result from their physical properties, especially in the depth dose distribution. The energy deposited in the tissue increases non-linearly; as the kinetic energy of the charged particles decreases, it reaches an explosive maximum ("Bragg peak") at a depth dependent on the initial energy, followed by a steep drop in dose (Fig. 1).
The LET capacity for protons is low and is around 0.5 keV/µm at the beginning of the travel distance in the tissue, where the velocity is highest. However, on the last µm of the range it reaches a maximum of approx. 100 keV/µm. For helium ions, the maximum is reached at approx. 200 keV/µm. In order to be able to irradiate a tumor with a given extent, the entrance energy is modulated in such a way that a spectrum results whose Bragg peaks are evenly distributed over the target volume, whereby a homogeneous dose distribution is achieved in the target volume (Fig. 1). This is called a spread-out Bragg-Peak (SOBP). The depth dose distribution of a monoenergetic proton beam looks extremely attractive, very low dose prior to the Bragg-Peak and no dose at all behind. However, the sum of different energies to cover the entire tumor also add up to increase the entrance dose. Moreover, protons lack the build -up effect therefore the dose to the superficial 3–5 mm of skin can be higher than that of photons. The dose to skin of a 15 MV photon beam is about 30% of the maximum dose, the dose to skin of a proton beam to treat a larger, deep-seated tumor is between 60 and 80% of the maximum dose. A clear advantage of protons is the steep drop in dose after the Bragg peak and the low lateral penumbra, both of which lead to a reduction in dose in normal tissues. The path of helium ions in tissue at depth is remarkably straighter compared to protons and this explains the increasing interest in this particle for a further optimized dose distribution compared to protons.
Carbon ions were introduced into clinical practice in 1994 by the National Institute of Radiological Sciences (NIRS) in Chiba, Japan, with the Heavy Ion Medical Accelerator (HIMAC). Carbon nuclei are 12 times heavier than protons and have a high LET value, which makes them a fascinating modality for the treatment of large, hypoxic and radioresistant tumors. Moreover, carbon ion LET can be considered high only in the last portion of their path so they can be used to apply high LET radiation in the target volume without exposing the uninvolved tissue in the entrance channel to high LET. Compared to fast neutron therapy, which was the first high-LET radiation modality used to treat cancer [3], the dose distribution can be applied with higher precision. Compared to protons and helium ions, the depth dose distribution of carbon ions is more complex and the end of the range after the SOBP is less precise due to the fragmentation of some of these large particles into smaller ions. The LET of a carbon ion beam is different from one voxel to another, which explains the need for using models to calculate the applied dose. Unfortunately, different models are used for calculating the RBE-weighted dose which make it difficult to compare the dose values reported in the different countries [4, 5]. Currently carbon ion beams are available in only few countries (South-East Asia and Europe). They have been used with excellent results in many tumors where local control was difficult to achieve with other modalities [6, 7].
For clinical applications, 2 different qualities of neutrons must be distinguished by their kinetic energies, which lead to different interactions with matter. Fast neutrons (for medical purposes in the range of 1 MeV -18 MeV) essentially interact through elastic and inelastic scattering. The effect of elastically scattered neutrons on the water molecules in tissue produces recoil protons, which in turn represent the ionizing radiation that causes biological effects. Thermal neutrons (E ≤ 0.5 eV) lead to nuclear reactions. Both neutron qualities are used for cancer treatment Fast Neutron Therapy (FNT) and Boron Neutron Capture Therapy (BNCT) respectively. Already shortly after the discovery of the neutron by Chadwick in 1932 [8], their use against cancer has been proposed. Stone and Larking carried out the first treatments with FNT as early as 1938, which led to poor results [9, 10]. After very extensive radiation biological investigations, FNT restarted in the 1960s with great enthusiasm and amazing good tumor response [11]. The challenge in fast neutron therapy was the selection of the tumor entities that are expected to benefit from the differential action of high LET radiation on tissues. An evidence-based advantage of FNT over low LET irradiation has been established in clinical trials for well-differentiated salivary gland tumors [11]. However, an increased appearance of unexpected late effects on normal tissues observed after an observation period of some years was used as an argument against FNT [3]. These late severe toxicities were controlled by advanced treatment planning and optimized beam delivery (e.g. isocentric gantries, multileaf collimators, intensity modulated fast neutron therapy). Nevertheless, the bad reputation of fast neutrons was overruling the good results obtained by a rapidly decreasing number of centers and carbon ions are replacing them [12, 13]. Nowadays only one place in the world offers a fast neutron beam fulfilling all modern requirements (University of Washington Medical Cyclotron Facility).
Thermal neutrons are needed for Boron Neutron Capture Therapy (BNCT). The basic idea to use neutron capture reactions in cancer treatment was published by Locher in 1936 [14]. Early BNCT trials in the 1950ies also were not leading to clinical success [15]. After successful pioneering work in Japan by Hiroshi Hatanaka using thermal neutrons at a research reactor for intraoperative BNCT [16, 17] international efforts restarted [16]. Despite promising clinical observations, the impact of BNCT has so far remained low, as the required radiation quality was only available at research reactors [18]. Recent developments in accelerator technology are now making BNCT facilities available in hospitals, marking a new and hopefully successful start for this auspicious modality. We are observing a rapidly growing number of such systems and hope to see the results of modern controlled, prospective clinical studies.
4 How does BNCT work?
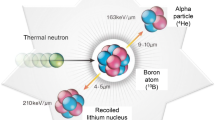
BNCT requires two independent agents: a high-intensity neutron beam with low energy ("epithermal") and a drug containing the non-radioactive isotope 10B which preferentially accumulates in the tumor cells. Neither of these alone has any effect on the tumor. BNCT utilizes the high probability of the non-radioactive nuclide 10B to capture thermal neutrons (cross section σth 3,835 b), which leads to the prompt nuclear reaction 10B(n,α)7Li. The products of this reaction have high LET characteristics (α particle approximately 150 keVµm−1, 7Li-nucleus approximately 175 keVµm−1) [15]. The path lengths of these particles are in the range of 4.5 µm to 10 µm resulting in an energy deposition limited to the diameter of a single cell. This makes it possible to selectively irradiate tumor cells that have absorbed a sufficient amount of 10B while sparing normal cells in the immediate vicinity, which contain less 10B. BNCT has two different targeting mechanisms that work independently of each other. On the one hand, the drug is transported to the tumor by biological systems, but it is not itself damaging to cells. The neutrons are directed to the tumor area by exposing it directly to the neutron field. Even a very high concentration of 10B in an organ outside the tumor (e.g. in the kidneys, if the drug is excreted via the urine) does not damage this organ. This is a completely different situation from targeted therapies with radioactive isotopes.
5 Why could BNCT represent a paradigm shift in radiation oncology?
There is an inherent problem with all current therapy techniques in radiation oncology: The target that has to be destroyed is always defined as a volume of tissue. Normal cells within the target volume also receive the full dose and will be destroyed. Outside the target volume, there are onset/decay areas, where a biologically relevant dose is delivered to normal tissues. Imaging techniques can only reflect partial aspects of reality and the target volume will vary depending on the imaging modalities available. Ultimately, a physician must determine the volume to be treated, which will vary between individuals, but also depending on daily performance. Time is another factor that plays an important role. The target will change location and size over time. Even the most advanced conventional external beam radiotherapy with photons or particles, including adaptive radiotherapy, or alternative approaches such as brachytherapy or intraoperative radiation therapy, are means to mitigate these facts, but they cannot eliminate them. BNCT represents a type of “disease-targeted” therapy: The treated volume is determined at the biological level and the treatment only damages cancer cells wherever they are located, but not the normal tissue. The particles responsible for the effect of BNCT irradiation have a high LET value, but they are only released in cancer cells. This makes it possible to successfully treat radiation-resistant tumors without causing severe side effects to healthy structures, as was the case in the early days of fast neutron therapy, for example. The protection of normal cells by BNCT in a volume irradiated with high dose, makes it possible to re-irradiate an area, where a local recurrence occurs after high dose radiotherapy. Early clinical results strongly support this hypothesis [19,20,21,22].
While great progress has been made over the last decade in the development of accelerator-based neutron sources to replace research reactors, the pharmaceutical industry has not been interested in investing in the development of boron carriers. Although many boron compounds were synthesized that could be used for BNCT, in no case was a real drug development successfully completed. Since the end of the 1960s, therefore, only two compounds (BPA and BSH) have been available for the treatment of patients with BNCT [23]. There is a lot of catching up to do here, although the first promising results in terms of industry-sponsored drug development are available [24, 25].
In summary, it can be stated that with the new technical possibilities (accelerator-based systems), a neutron beam with high intensity and low energy for BNCT can be integrated into a hospital and thus a new and promising therapy modality becomes available for everyday clinical practice. This new therapy promises a solution for clinical problems that currently cannot be treated or can only be treated with little success.
References
Okun LB. The Theory of Weak Interaction. In: Prentki J, editor. Proceedings of the 1962 International conference on high-energy Physics, CERN, July 4–11. Geneva, Switzerland; 1962. p. 845 Bibcode:1962hep..conf..845O.
The NIST Reference on Constants, Units, and Uncertainty. National Institute of Standards and Technology. 2018. 018 CODATA Value: electron mass. Retrieved 2024–01–10
Jones B. Clinical radiobiology of fast neutron therapy: what was learnt? Front Oncol. 2020;10:1537. https://doi.org/10.3389/fonc.2020.01537.
Fossati P, Molinelli S, Matsufuji N, Ciocca M, Mirandola A, Mairani A, et al. Dose prescription in carbon ion radiotherapy: a planning study to compare NIRS and LEM approaches with a clinically-oriented strategy. Phys Med Biol. 2012;57(22):7543–54.
Chanrion M-A, Sauerwein W, Jelen U, Wittig A, Engenhart-Cabillic R, Beuve M, M. The influence of the local effect model parameters on the prediction of the tumor control probability for prostate cancer. Phys Med Biol. 2014;59:3019–40. https://doi.org/10.1088/0031-9155/59/12/3019.
Orlandi E, Barcellini A, Vischioni B, Fiore MR, Vitolo V, Iannalfi A, et al. The role of carbon ion therapy in the changing oncology landscape-a narrative review of the literature and the decade of carbon ion experience at the Italian national center for oncological hadrontherapy. Cancers (Basel). 2023;15(20):5068. https://doi.org/10.3390/cancers15205068.
Holtzman AL, Seidensaal K, Iannalfi A, Kim KH, Koto M, Yang WC, et al. Carbon ion radiotherapy: an evidence-based review and summary recommendations of clinical outcomes for skull-base chordomas and chondrosarcomas. Cancers (Basel). 2013;15(20):5021. https://doi.org/10.3390/cancers15205021. PMID: 37894388; PMCID: PMC10605639.
Chadwick J. The existence of a neutron. Proc of the Royal Society of London. 1932;136:692–708.
Stone RS, Larkin JC. The treatment of cancer with fast neutrons. Radiology. 1942;39:608–20.
Stone RS, Lawrence JH, Aebersold PC. A preliminary report on the use of fast neutrons in the treatment of malignant disease. Radiology. 1940;35:322–7.
Catterall M. The treatment of advanced cancer by fast neutrons from the Medical Research Council’s cyclotron at Hammersmith Hospital. London Eur J Cancer. 1974;10(6):343–7.
Laramore GE, Krall JM, Griffin TW, Duncan W, Richter MP, Saroja KR, et al. Neutron versus photon irradiation for unresectable salivary gland tumors: final report of an RTOG-MRC randomized clinical trial radiation therapy oncology group medical research council. Int J Radiat Oncol Biol Phys. 1993;27(2):235–40.
Schulz-Ertner D, Nikoghosyan A, Didinger B, Münter M, Jäkel O, Karger CP, Debus J. Therapy strategies for locally advanced adenoid cystic carcinomas using modern radiation therapy techniques. Cancer. 2005;104(2):338–44.
Stannard C, Vernimmen F, Carrara H, Jones D, Fredericks S, Hille J, de Kock E. Malignant salivary gland tumours: can fast neutron therapy results point the way to carbon ion therapy? Radiother Oncol. 2013;109(2):262–8.
Locher GL. Biological effects and therapeutic possibilities of neutrons. Am J Roentgenol Radium Ther. 1936;36(1):1–13.
Sauerwein W. Principles and roots of neutron capture therapy. In: Sauerwein W, Wittig A, Moss R, Nakagawa Y, editors. In neutron capture therapy principles and applications. Heidelberg New York Dordrecht London: Springer; 2012. p. 1–16. https://doi.org/10.1007/978-3-642-31334-9.
Hatanaka H. A revised boron-neutron capture therapy for malignant brain tumors II Interim clinical result with the patients excluding previous treatments. J Neurol. 1975;209(2):81–94.
Hatanaka H. Clinical results of boron neutron capture therapy. Basic Life Sci. 1990;54(15):15–21.
Sauerwein W., Moss R. (eds) 2009: Requirements for Boron Neutron Capture Therapy (BNCT) at a nuclear research reactor. EUR 2383 EN. Luxembourg: office for official publications of the European commission. EUR - Scientific and Technical Research series - ISSN 1018–5593 ISBN 978–92–79–12431–0 https://doi.org/10.2790/11743.
Kankaanranta L, Seppälä T, Koivunoro H, Saarilahti K, Atula T, Collan J, et al. Boron neutron capture therapy in the treatment of locally recurred head-and-neck cancer: final analysis of a phase I/II trial. IJROBP. 2012;82(1):e67–75.
Koivunoro H, Kankaanranta L, Seppala T, Haapaniemi A, Makitie A, Joensuu H. Boron neutron capture therapy for locally recurrent head and neck squamous cell carcinoma: An analysis of dose response and survival. Radiother Oncol. 2019;137:153–8.
Wang LW, Chen YW, Ho CY, Hsueh Liu YW, Chou FI, Liu YH, et al. Fractionated boron neutron capture therapy in locally recurrent head and neck cancer: a prospective phase I/II trial. Int J Radiat Oncol Biol Phys. 2016;95(1):396–403.
Sauerwein W, Bet P, Wittig A. Drugs for BNCT: BSH and BPA. In: Sauerwein W, Wittig A, Moss R, Nakagawa Y, editors. In neutron capture therapy principles and applications. Heidelberg New York Dordrecht London: Springer; 2012. p. 117–60. https://doi.org/10.1007/978-3-642-31334-9.
Sauerwein WAG, Sancey L, Hey-Hawkins E, Kellert M, Panza L, Imperio D, et al. Theranostics in boron neutron capture therapy. Life. 2021;11(4):330. https://doi.org/10.3390/life11040330.
Raitano A, Martin T, Zhang C, Malinao M-C, Capo L, Ikeura M, et al. Boronotyrosine, a Borylated amino acid mimetic with enhanced solubility, tumor boron delivery, and retention for the reemerging boron neutron capture therapy field. J Med Chem. 2023;66:13809–20.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Wolfgang Sauerwein, Joël Hérault, Raymond Moss, Piero Fossati, Saverio Altieri, Andrea Wittig, and Kazuyo Igawa. The measurements for Fig. 1 were performed by Joël Hérault. The first draft of the manuscript was written by Wolfgang Sauerwein and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Financial interests
Wolfgang Sauerwein is co-founder of the company BNCT Global GmbH . All other authors have no relevant financial or non-financial interests to disclose.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sauerwein, W.A.G., Igawa, K., Herault, J. et al. Hadron therapy in radiation oncology and why BNCT is a paradigm shift. Health Technol. (2024). https://doi.org/10.1007/s12553-024-00848-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12553-024-00848-5